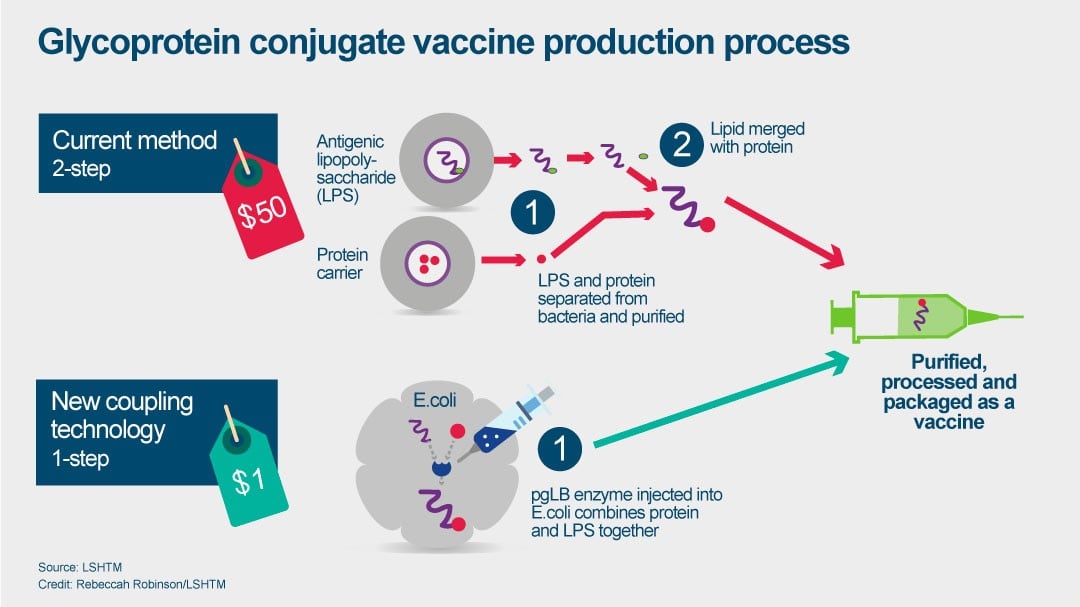
Vaccine Production Process Diagram, Every Vaccine And Treatment In Development For Covid 19 So Far
Vaccine production process diagram Indeed recently is being sought by users around us, perhaps one of you. People are now accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the title of this post I will discuss about Vaccine Production Process Diagram.
- Chapter 79 Pharmaceutical Industry
- Developing Covid 19 Vaccines At Pandemic Speed Nejm
- Surveillance Manual Tetanus Vaccine Preventable Diseases Cdc
- The Promise Of Mrna Vaccines A Biotech And Industrial Perspective Npj Vaccines
- Recombinant Vaccine
- Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International
Find, Read, And Discover Vaccine Production Process Diagram, Such Us:
- Developing Covid 19 Vaccines At Pandemic Speed Nejm
- Production Of Hepatitis B Vaccine Its Process With Diagram
- Chapter 79 Pharmaceutical Industry
- Developing And Manufacturing Attenuated Live Bacterial Vaccines Biopharm International
- Current And Emerging Cell Culture Manufacturing Technologies For Influenza Vaccines
If you re looking for Mmr Vaccine Brand Name Canada you've come to the right place. We ve got 104 graphics about mmr vaccine brand name canada adding images, photos, photographs, wallpapers, and much more. In these webpage, we also provide variety of graphics available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, translucent, etc.
Diagram Purpose Of Process Flow Diagram Full Version Hd Quality Flow Diagram Elkwiringharness Amichediviaggio It Mmr Vaccine Brand Name Canada
There should be complete validation of manufacturing processes to ensure the continuous conformity of vaccine batches to required standards ec 1990a.
Mmr vaccine brand name canada. The discovery that dcs can be generated from monocytes or cd34 precursors permitted harvesting of these rare cells in large numbers for. Until recently this production process also began with egg grown cvvs per fda regulations. Stages of vaccine production vaccine production has several stages.
A diagram of the progression of product and pr ocess development. Vaccine manufacturers apply to the fda for a license to manufacture a vaccine by submitting a product license application pla. Antonia in gene therapy of cancer third edition 2014.
Inactivation this involves making of the antigen preparation purification the isolated antigen is purified formulation the purified antigen is combined with. This large scale production is often a challenge. The production of a vaccine can take between 6 and 36 months vaccines manufacturing is a biological process where a very high level of expertise is required.
There also is a cell based production process for flu vaccines that was approved by fda in 2012. A general flow diagram of a purification train in the vaccine production process paul k ng. The nal vaccine manufacturing process and must embody the.
Inactivation of microorganism 22. This production method requires large numbers of chicken eggs to produce vaccine and may take longer than other production methods. Viruses can be lipid coatedenveloped or non enveloped.
Ples that any procedure process equip ment material activity or system leads to the results expected. We need to continually adapt production process to satisfy evolving regulatory demand which varies country by country. The first step in ex vivo vaccine production is the generation of an enriched population of mature dendritic cells for vaccines.
From laboratory method and phase i trials. Methods thatensure product quality and standards for the. The pla describes the firms vaccine manufacturing process quality control and the results of clinical studies documenting the vaccines safety and efficacy.
More From Mmr Vaccine Brand Name Canada
- Influenza Vaccine Manufacturers In India
- Vaccine Failures In History
- Down Syndrome Black Sphynx Cat
- Vaccine News Coronavirus
- Pfizer Chief Politicization Vaccine Development
Incoming Search Terms:
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctp Jbfq K3iwac1mhcwxqpxtkqslgg9rruhfoggfzcaqwxtykf Usqp Cau Pfizer Chief Politicization Vaccine Development,
- Mrna As A Transformative Technology For Vaccine Development To Control Infectious Diseases Sciencedirect Pfizer Chief Politicization Vaccine Development,
- Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International Pfizer Chief Politicization Vaccine Development,
- Covid 19 Halix Ready For Vaccine Production European Biotechnology Pfizer Chief Politicization Vaccine Development,
- Recombinant Vaccine Pfizer Chief Politicization Vaccine Development,
- Optimizing The Utilization Of Aluminum Adjuvants In Vaccines You Might Just Get What You Want Npj Vaccines Pfizer Chief Politicization Vaccine Development,