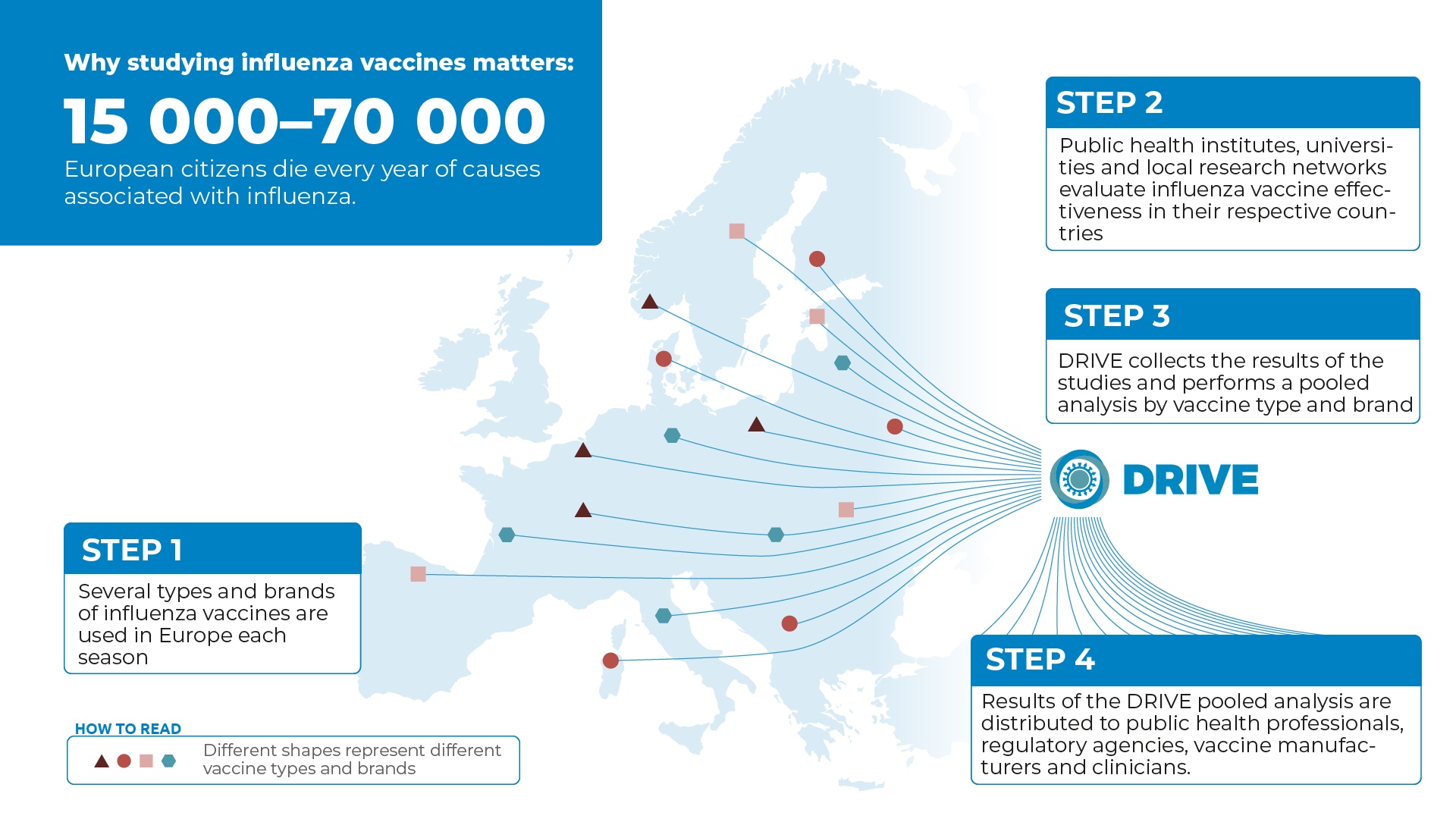
Influenza Vaccine Manufacturing Process, Effectiveness Of The Seasonal Flu Vaccine And New Production Methods Infographic Pharmaceutical Journal
Influenza vaccine manufacturing process Indeed lately is being hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the net in gadgets to view video and image data for inspiration, and according to the name of the article I will talk about about Influenza Vaccine Manufacturing Process.
- Mutation In Egg Based Production Renders This Year S Flu Vaccine Less Effective
- Http Www Vaccineplace Com Docs H1n1productionprocess Pdf
- Influenza Tk Talk
- Vaccines Free Full Text Egg Independent Influenza Vaccines And Vaccine Candidates Html
- Development Of Production And Purification Platformform For Influenza
- Full Article Cell Culture Derived Flu Vaccine Present And Future
Find, Read, And Discover Influenza Vaccine Manufacturing Process, Such Us:
- Developing Covid 19 Vaccines At Pandemic Speed Nejm
- Http Www Vaccineplace Com Docs H1n1productionprocess Pdf
- Development Of Production And Purification Platformform For Influenza
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcreabxoanhcfz9bik0adxogu1n8uhwqug0zkyx9zi0 Usqp Cau
- Assessment Of An Optimized Manufacturing Process For Inactivated Quadrivalent Influenza Vaccine A Phase Iii Randomized Double Blind Safety And Immunogenicity Study In Children And Adults Bmc Infectious Diseases Full Text
If you are searching for Pneumococcal Vaccine Singapore Moh Guidelines you've come to the ideal place. We have 104 images about pneumococcal vaccine singapore moh guidelines adding pictures, photos, pictures, backgrounds, and much more. In these page, we additionally have number of graphics available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, transparent, etc.

Egg Based Pilot Influenza Vaccine Production Process Download Scientific Diagram Pneumococcal Vaccine Singapore Moh Guidelines
The nal vaccine manufacturing process and must embody the.
Pneumococcal vaccine singapore moh guidelines. However sometimes an existing but unexpected flu strain will become prevalent during flu season making the vaccine a. Regulatory agencies in some countries may require clinical testing before approving the vaccine which adds to the time before the vaccine is availablethe full process in a best case. The manufacturing process continues with quality testing filling and distribution.
If the vaccine is made with the same processes as the seasonal influenza vaccine and in the same manufacturing plant this can be very rapid one to two days. We need to continually adapt production process to satisfy evolving regulatory demand which varies country by country. Formal who recommendations were first issued in 1973.
Fda tests and approves all influenza vaccines prior to release and shipment. Inoculation of 11 days old embryonated eggs local supplier romania with influenza seed virus influenza ah3n2 of strain auruguay7162007 x 175c solvay weesp the netherlands incubation of inoculated eggs for 72 hr at 350c and overnight. For the nasal spray flu vaccine ie the live attenuated influenza vaccine laiv the starting cvvs are live but weakened viruses that go through a different production process.
There are entire chicken farms in the us and around the world dedicated to producing eggs for the purpose of incubating influenza viruses for use in vaccines. The production of a vaccine can take between 6 and 36 months vaccines manufacturing is a biological process where a very high level of expertise is required. Cell based influenza virus production process using single use equipment.
The most striking feature of the h1n1 flu vaccine manufacturing process is the 1200000000 chicken eggs required to make the 3 billion doses of vaccine that may be required worldwide. Science behind the vaccine. The most common method used to produce each years seasonal flu vaccine involves a laborious time consuming process in which scientists must select vaccine strains months in advance of the upcoming flu season and then grow the selected flu virus strains in chicken eggs.
E long lead times in man ufactur ing process design facility implementation and start up ne. The aim of this article is to demonstrate how cytiva single use products can be applied in the field of vaccine manufacturingincluding a brief discussion around modern vaccine processes followed by a case study showing the scale up of upstream and downstream processes for the production of a cell based live attenuated.
More From Pneumococcal Vaccine Singapore Moh Guidelines
- Down Syndrome Awareness Month Day 27
- Pfizer Covid 19 Vaccine Trial Side Effects
- China Vaccine Meme
- Pfizer Stock Price Today Yahoo
- Pfizer Covid Vaccine Sign Up
Incoming Search Terms:
- Https Www Ema Europa Eu En Documents Scientific Guideline Guideline Influenza Vaccines Quality Module Revision 1 En Pdf Pfizer Covid Vaccine Sign Up,
- Emerging Technologies For Low Cost Rapid Vaccine Manufacture Kis 2019 Biotechnology Journal Wiley Online Library Pfizer Covid Vaccine Sign Up,
- Membrane Based Clarification Of Polysaccharide Vaccines Bioprocess Internationalbioprocess International Pfizer Covid Vaccine Sign Up,
- Advances In The Development Of Influenza Virus Vaccines Nature Reviews Drug Discovery Pfizer Covid Vaccine Sign Up,
- Https Www Bio Fiocruz Br En Images Stories Pdfs Mpti 2013 Selecao Vaccine Process Technology Pdf Pfizer Covid Vaccine Sign Up,
- Operations Management Everyday Challenges And Opportunities Have You Gotten Your Flu Shot Yet Pfizer Covid Vaccine Sign Up,