Vaccine Phase 3 Trial Duration, Phase 3 Trial Of Sinovac S Covid 19 Vaccine Begins
Vaccine phase 3 trial duration Indeed recently has been hunted by users around us, perhaps one of you. People now are accustomed to using the net in gadgets to see image and video information for inspiration, and according to the name of the article I will talk about about Vaccine Phase 3 Trial Duration.
- The Main Event Moderna Begins Final Phase Of Covid 19 Vaccine Study
- Phase Iii Testing Of Astrazeneca Covid 19 Vaccine Candidate Begins Technology Networks
- Coronavirus Oxford Vaccine Trials To Resume In U K The Hindu
- Covid 19 Vaccine Developed By Us Biotech Firm Moderna Enters Final Stage Trial
- Russia To Start Covid 19 Vaccine Phase 3 Trials In Aug Teletrader Com
- Here S What It Actually Means If A Covid 19 Vaccine Passes Phase 3 Clinical Trials
Find, Read, And Discover Vaccine Phase 3 Trial Duration, Such Us:
- Uabg5zptxo37hm
- Yale And Yale New Haven Hospital Begin Phase 3 Trial Of Covid 19 Vaccine Yalenews
- Sanofi Gsk Start Phase 1 2 Clinical Trial Of Covid 19 Vaccine Candidate
- Phase 3 Trial Of Sinovac S Covid 19 Vaccine Begins
- Why Are Children Excluded From Phase 3 Clinical Trials
If you are looking for Pfizer Stock Watch you've arrived at the perfect place. We have 100 graphics about pfizer stock watch including pictures, pictures, photos, backgrounds, and more. In such web page, we also provide number of graphics available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.
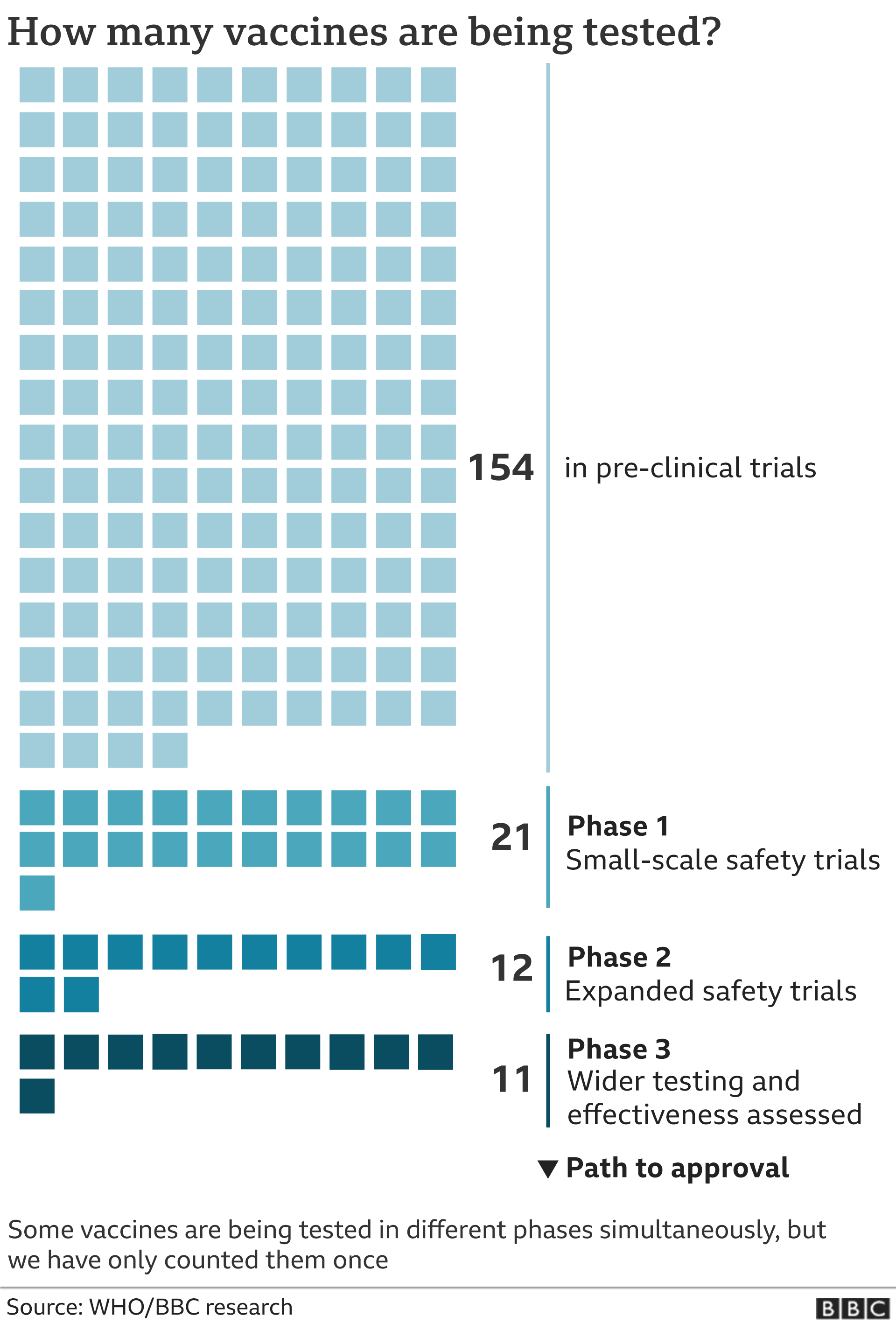
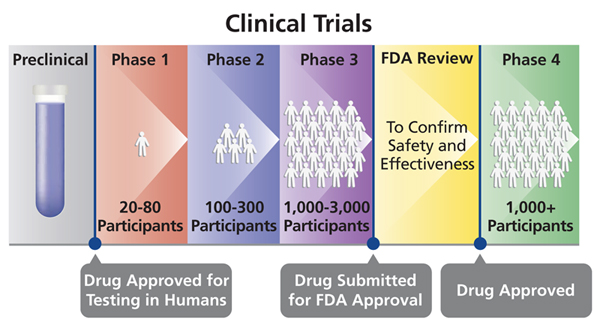
The fda usually requires a phase iii clinical trial before approving a new medication.
Pfizer stock watch. In phase ii the clinical study is expanded and vaccine is given to people who have characteristics such as age and physical health similar to those for whom the new vaccine is intended. Phases 1 and 2 which typically involve giving the drug to smaller groups of volunteers aim to show. A vaccine trial is a clinical trial that aims at establishing the safety and efficacy of a vaccine prior to it being licensed.
Mva elicited immune responses similar to those associated. The late stage trial will include 30000 participants and is expected. O n june 11 biotech company moderna announced it had finalized plans for phase 3 testing of its covid 19 vaccine candidate.
Due to the larger number of participants and longer duration or phase iii rare and long term side effects. Volunteer participating in phase 3 trial of the sinovac covid 19 vaccine in padjadjaran university bandung west java indonesia. A phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 covid 19 in adults has begun.
The 2 delays follow a similar incident with phase 3 trials of a vaccine being jointly worked on by oxford university and astra zeneca which was briefly delayed last month due to an unexplained illness in one participant. In phase iii the vaccine is given to thousands of people and tested for efficacy and safety. Trials of that vaccine have now resumed globally with the exception of the us for reasons unknown.
Maj gen ikram said it was an honour for the country that it was participating in the phase 3 trial of a vaccine for the first time. More than 60000 chinese citizens have received the potential vaccines in phase 3 trials. A phase 3 trial is usually a drug or vaccines final test before it can be sold to the public.
During phase i small groups of people receive the trial vaccine. The vaccine known as mrna 1273 was co developed by the cambridge massachusetts based biotechnology company moderna inc and the national institute of allergy and infectious diseases.
More From Pfizer Stock Watch
- Hep B Vaccine Box
- Covid Vaccine Trials Houston
- Vaccine Gun Price In Bangladesh
- Vaccine Clipart Free
- Vaccine Mechanism Of Action
Incoming Search Terms:
- Coronavirus Oxford Vaccine Trials To Resume In U K The Hindu Vaccine Mechanism Of Action,
- China Has 11 Covid 19 Vaccines In Clinical Trials Four In Phase Iii Cgtn Vaccine Mechanism Of Action,
- Az Expands Phase 3 Trials Of Covid 19 Vaccine Frontrunner Into Us Vaccine Mechanism Of Action,
- Astrazeneca S Coronavirus Vaccine Has Reached Phase 3 Clinical Trials In Us Good News News India Tv Vaccine Mechanism Of Action,
- Covid 19 Vaccine How Much Time Vaccine Development Takes Stages And Timeline Explained The Financial Express Vaccine Mechanism Of Action,
- What To Expect As Moderna S Covid 19 Vaccine Moves To Phase 3 Trials Vaccine Mechanism Of Action,