Vaccine Development Phases Duration, The Development And Lifespan Of A Vaccine Passport Health
Vaccine development phases duration Indeed lately has been hunted by users around us, maybe one of you personally. People are now accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this post I will discuss about Vaccine Development Phases Duration.
- Pipeline Of Vaccines Tbvi
- How Long Does It Take To Develop A Vaccine World Economic Forum
- Safety And Immunogenicity Of The Chadox1 Ncov 19 Vaccine Against Sars Cov 2 A Preliminary Report Of A Phase 1 2 Single Blind Randomised Controlled Trial The Lancet
- U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
- On Pins And Needles Will Covid 19 Vaccines Save The World Mckinsey
- Vaccine Development Wur
Find, Read, And Discover Vaccine Development Phases Duration, Such Us:
- How Far Are We With The Accelerated Development Of Sars Cov 2 Vaccines A Review And Outlook Biopharma Excellence
- Vaccine Wikipedia
- Covid Have We Finally Got A Coronavirus Vaccine Bbc News
- 5 New Vaccine Development And The Future Needs Of The Special Immunizations Program Protecting The Frontline In Biodefense Research The Special Immunizations Program The National Academies Press
- Accelerating Vaccine Development And Manufacturing
If you are looking for Covid Vaccine Update Ireland you've reached the perfect place. We have 100 images about covid vaccine update ireland adding pictures, photos, pictures, wallpapers, and more. In such webpage, we additionally have variety of images out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
In phase iii the vaccine is given to.
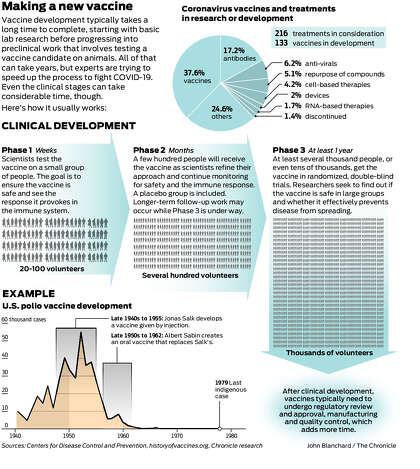
Covid vaccine update ireland. Here we discuss the key principles and challenges faced in the development of vaccines targeting a diverse set of pathogens from concept to clinical trial in humans. Vaccines typically take about 10 to 15 years to develop and approve through four phases that include human trials. Clinical development is a three phase process.
Discontinued projects were not included in this calculation since the decision can occur at any moment distorting results. With all eyes on covid 19 vaccine the national covid 19 task force experts team coordinator and spokesperson wiku adisasmito tried to explain the stages of vaccine development. Phase duration was determined by the average number of years a vaccine candidate takes to complete a development phase.
As with any drug clinical development of a vaccine takes place in three phases. During phase i small groups of people receive the trial vaccine. The first phase involves a relatively small group of healthy test subjects usually between 20 and 100 people.
But with operation warp speed approved vaccine projects can submit data to the. Currently a successful phase i safety clinical trial has been undertaken with a live attenuated chimeric virus based on the. Vaccine development has been on an accelerated track because of the perceived failure of anti mosquito interventions in limiting the incidence and geographic spread of wnv.
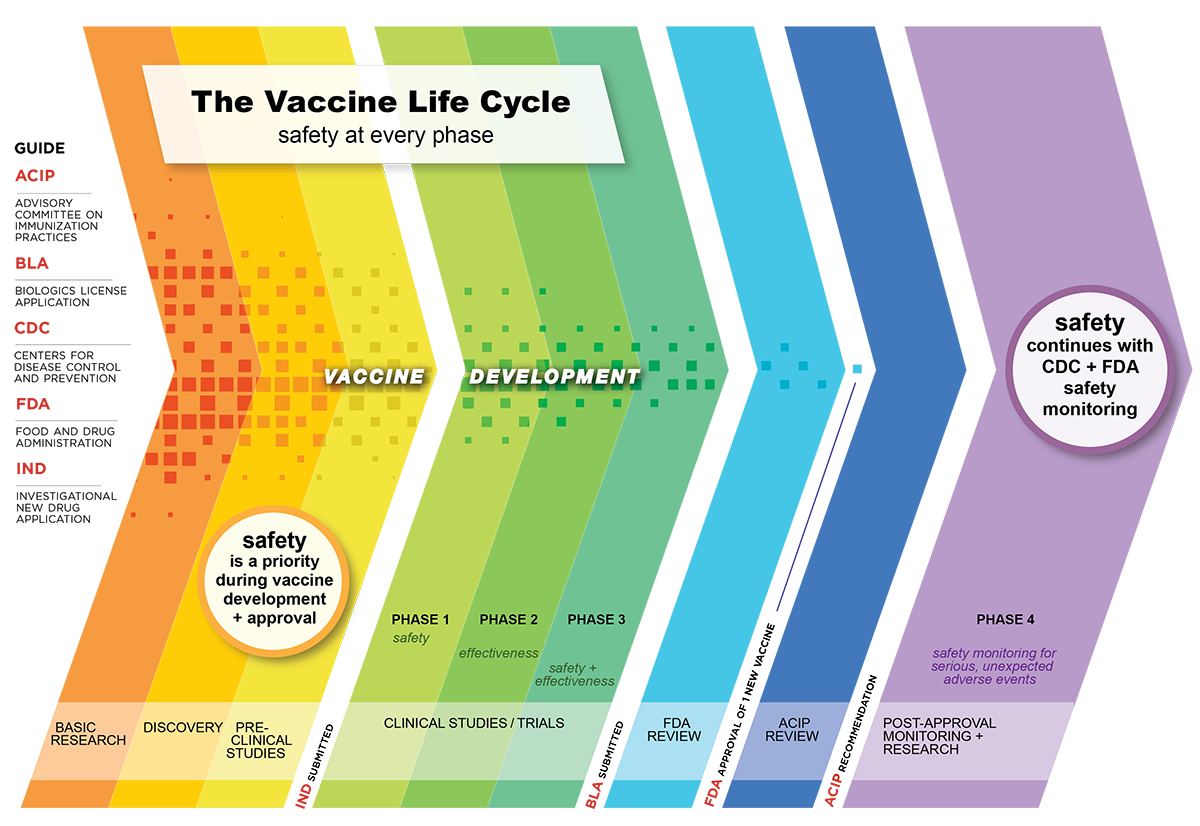
Basic research findings continue to inform the process of vaccine development even during clinical testing. For instance although basic research is the starting point it does not end when applied rd begins. The development cycle of a vaccine consists of six stages exploratory stage pre clinical stage clinical development regulatory review and approval and manufacturing quality control.
Ebel in international encyclopedia of public health 2008. 456 vaccine projects remain eligible. All the information and data collected during development and trials of a new vaccine are presented to relevant regulators at regional level where appropriate for example in the eu and national level.
In phase ii the clinical study is expanded and vaccine is given to people who have characteristics such as age and physical health similar to those for whom the new vaccine is intended. Regulatory approval submitting data and information to regulators to gain approval for vaccines.
Https Www Takedavaccines Com Siteassets Vaccines Behind Vaccine Development The Clinical Trial Process Infographic Final 100318 Pdf Covid Vaccine Update Ireland
More From Covid Vaccine Update Ireland
- Red Down Arrow Sign
- Flu Vaccine 202021 Uk Poster
- Tiger Down Syndrome Cow
- Covid Vaccine News Latest In Hindi
- Vaccine Development Phases
Incoming Search Terms:
- Every Vaccine And Treatment In Development For Covid 19 So Far Vaccine Development Phases,
- Vaccine Approaches In Early Phase Development Avac Vaccine Development Phases,
- Where Are We At With Developing A Vaccine For Coronavirus Vaccine Development Phases,
- Germany To Start First Coronavirus Vaccine Trial Germany News And In Depth Reporting From Berlin And Beyond Dw 22 04 2020 Vaccine Development Phases,
- Graphics How Soon Can We Get A Covid 19 Vaccine Cgtn Vaccine Development Phases,
- Vaccines Free Full Text Clinical Development Of A Cytomegalovirus Dna Vaccine From Product Concept To Pivotal Phase 3 Trial Vaccine Development Phases,