Vaccine Development Phases, Covid 19 Vaccine Research And Development Hillnotes
Vaccine development phases Indeed lately is being hunted by users around us, perhaps one of you personally. People now are accustomed to using the net in gadgets to view video and image information for inspiration, and according to the name of this post I will discuss about Vaccine Development Phases.
- Developing Covid 19 Vaccines At Pandemic Speed Nejm
- Developing Covid 19 Vaccines At Pandemic Speed Nejm
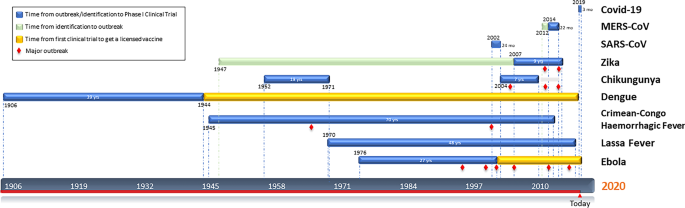
- Covid 19 Vaccines Breaking Record Times To First In Human Trials Npj Vaccines
- Why A Coronavirus Vaccine Takes So Long To Develop Business Insider
- Olcreate An Introduction To Coronavirus An Introduction To Coronavirus
- How Scientists Are Developing Coronavirus Vaccines At Speed
Find, Read, And Discover Vaccine Development Phases, Such Us:
- Covid 19 Vaccines Breaking Record Times To First In Human Trials Npj Vaccines
- The Covid 19 Vaccine Development Multiverse Nejm
- Immunogenicity And Safety Of A Recombinant Adenovirus Type 5 Vectored Covid 19 Vaccine In Healthy Adults Aged 18 Years Or Older A Randomised Double Blind Placebo Controlled Phase 2 Trial The Lancet
- U S Vaccine Safety Overview History And How It Works Cdc
- Vaccine Development For The Covid 19 National Biosafety Authority
If you re looking for Pfizer Vaccine Gates you've arrived at the ideal location. We ve got 100 images about pfizer vaccine gates adding pictures, photos, photographs, wallpapers, and more. In these webpage, we additionally provide variety of graphics out there. Such as png, jpg, animated gifs, pic art, logo, black and white, transparent, etc.
Matthew rozsa october 25 2020 1200pm utc articles about the ongoing effort to create a vaccine for the novel coronavirus often include talk of phases as if vaccine production were akin to.
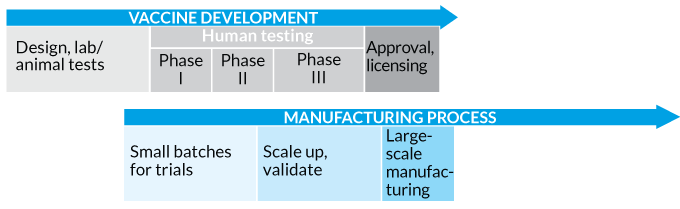
Pfizer vaccine gates. Our work to research develop and introduce a new vaccine typically goes through several stages. During phase i small groups of people receive the trial vaccine. Discovery using the isolated pathogen to develop a possible vaccine every disease has different characteristics so each vaccine will have an individual development path.
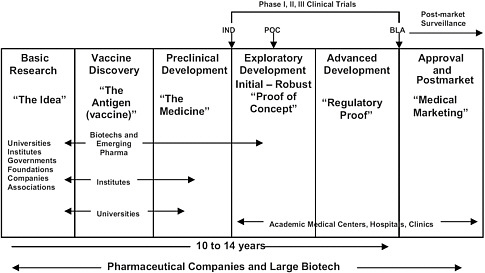
Basic research findings continue to inform the process of vaccine development even during clinical testing. Wiku said on thursday that vaccine development started with basic research also known as the exploratory phase. Phase 1 and 2a clinical trials normally last several months to even a year before proceeding to phase 2b or phase 3 trials in which the pool of people receiving the vaccine increases.
In phase iii the vaccine is given to. The purpose is to evaluate long term safety and efficacy identify rare adverse effects address relevant issues and determine potentials for. But with operation warp speed approved vaccine projects can submit data to the.
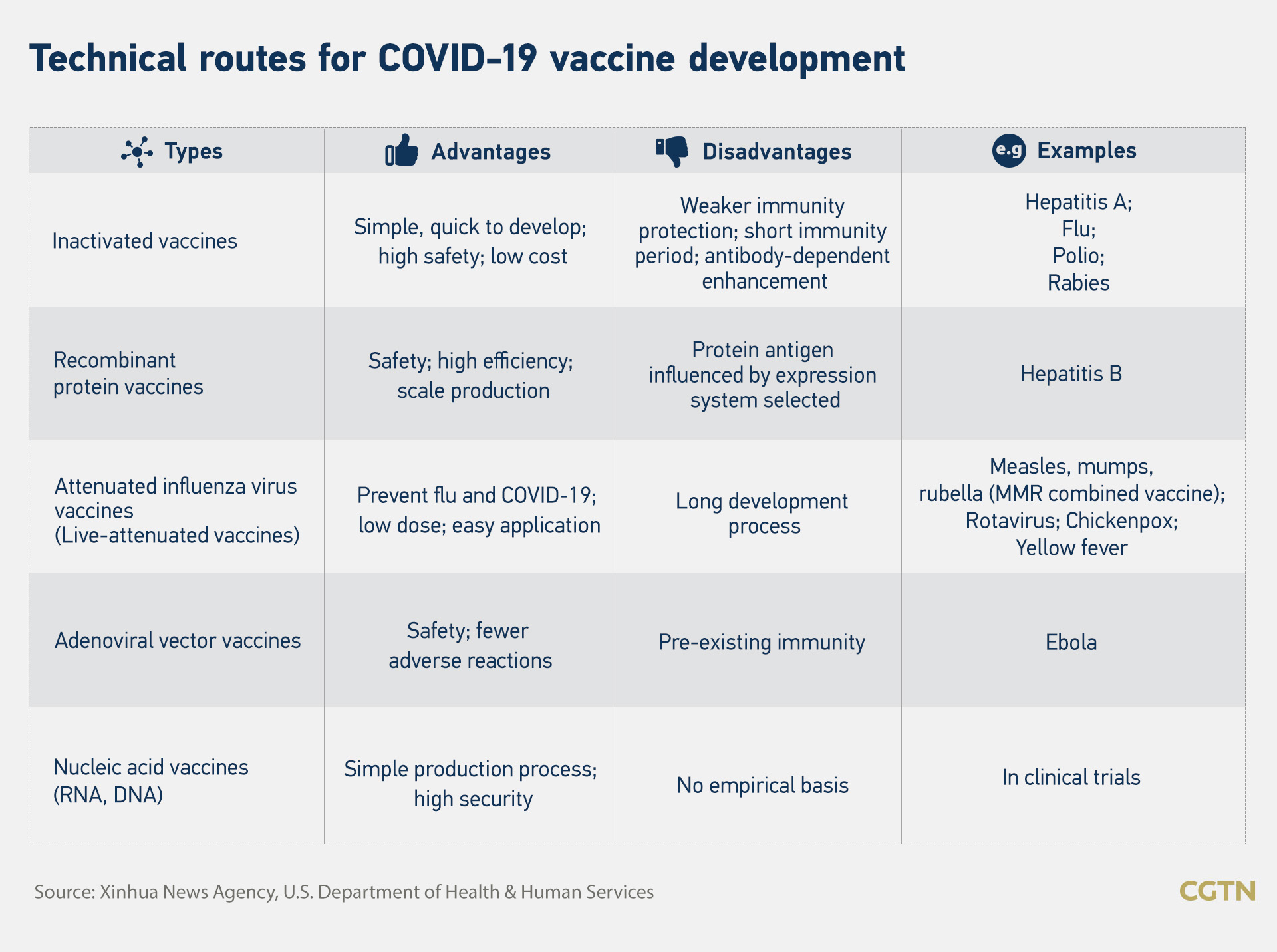
The development cycle of a vaccine consists of six stages exploratory stage pre clinical stage clinical development regulatory review and approval and manufacturing quality control. For instance although basic research is the starting point it does not end when applied rd begins. Knowledge of pathogen structure route of entry interaction with cellular receptors subsequent replication sites and disease causing mechanisms are all important to identify antigens suitable for.
Pathogen life cycle epidemiology. Clinical development is a three phase process. In reality the stages of vaccine development are not so neatly divided.
Phase iv or post marketing surveillance transpires after the vaccine has been licensed and administered to the public or more specifically within the quality control stage of vaccine development. The stage features basic lab research that focuses on examining the virus and the. Any new vaccine development is greatly enhanced by and requires integration of information concerning.
More From Pfizer Vaccine Gates
- Apple Stock Price Prediction
- Coronavirus Vaccine News Russian
- Covid Vaccine Human Trials India
- Vaccine Ready Date
- Down Syndrome Awareness Day 7
Incoming Search Terms:
- Coronavirus Researchers Compete To Enroll Subjects For Vaccine Tests Wsj Down Syndrome Awareness Day 7,
- India Eyes Global Front Runners In Covid 19 Vaccine Plan India News Hindustan Times Down Syndrome Awareness Day 7,
- Https Www Cfr Org Backgrounder What World Doing Create Covid 19 Vaccine Down Syndrome Awareness Day 7,
- How Can We Develop A Covid 19 Vaccine Quickly News Wellcome Down Syndrome Awareness Day 7,
- Opinion How Long Will A Vaccine Really Take The New York Times Down Syndrome Awareness Day 7,
- How Are Vaccines Developed Pfizer Down Syndrome Awareness Day 7,