Vaccine Research Phases, Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html
Vaccine research phases Indeed lately is being hunted by consumers around us, perhaps one of you. People now are accustomed to using the net in gadgets to view video and image information for inspiration, and according to the name of the article I will talk about about Vaccine Research Phases.
- Covid Have We Finally Got A Coronavirus Vaccine Bbc News
- The Development And Lifespan Of A Vaccine Passport Health
- How We Develop New Vaccines Gsk
- As We Await A Vaccine These Covid 19 Treatments Are Being Tested Science News
- First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
- How Scientists Are Developing Coronavirus Vaccines At Speed
Find, Read, And Discover Vaccine Research Phases, Such Us:
- U S Advisory Group Lays Out Proposal On How To Prioritize Covid 19 Vaccine
- Accelerating Vaccine Development And Manufacturing
- Coronavirus Covid 19 Vaccine Covaxin Corona Vaccine India Update What And How Long Does It Take To Make A Vaccine What S The Covid Timeline
- Estimating The Cost Of Vaccine Development Against Epidemic Infectious Diseases A Cost Minimisation Study The Lancet Global Health
- U S Medical Drug Development Process Tradevistas
If you re searching for Pfizer Stock you've come to the perfect location. We have 100 graphics about pfizer stock adding pictures, photos, pictures, backgrounds, and more. In these page, we also have number of graphics available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, translucent, etc.
Moderna says it is on track to deliver at least 500 million doses per year beginning in 2021 thanks in part to the deal it has.

Pfizer stock. Researchers will be assessing the immune response to the. The vaccine known as mrna 1273 was co developed by the cambridge massachusetts based biotechnology company moderna inc and the national institute of allergy and infectious diseases. The science behind vaccine research and testing how vaccines are made and tested.
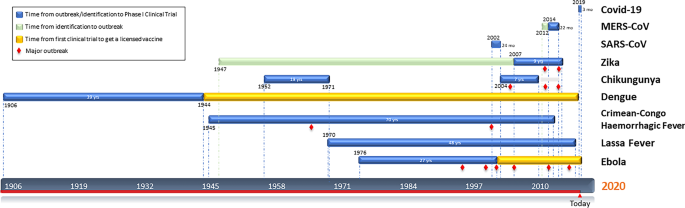
In this somewhat simplified view vaccine research begins only after a careful assessment of public health priorities. Phase three will test the vaccine in 30000 us. The creation of a vaccine involves scientists and medical experts from around the world and it usually requires 10 to 15 years of research before the vaccine is made available to the general public.
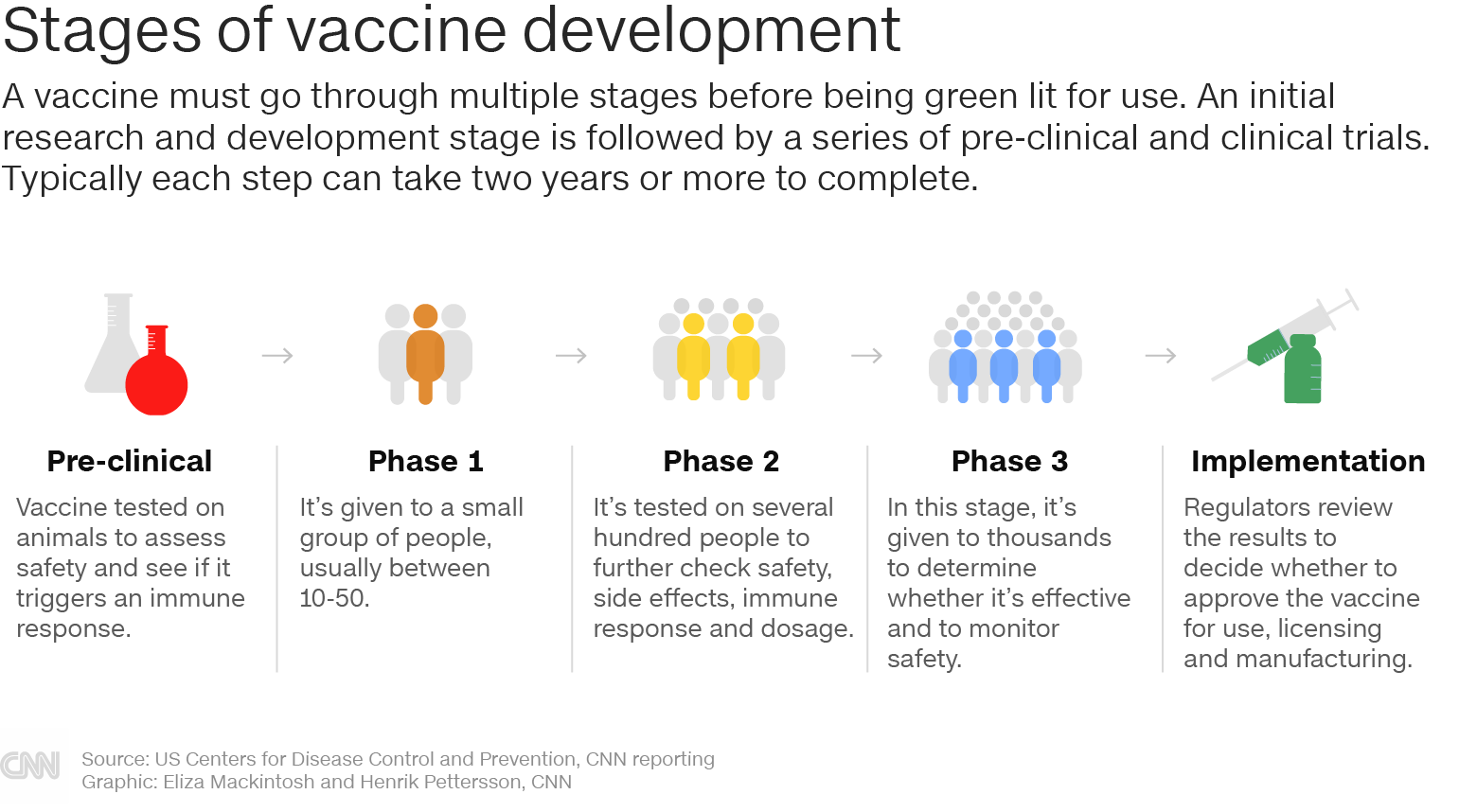
A phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 covid 19 in adults has begun. For the purposes of this chapter the process of vaccine research and development rd is described as if the process occurs in an ordered chronological fashion. In phase ii the clinical study is expanded and vaccine is given to people who have characteristics such as age and physical health similar to those for whom the new vaccine is intended.
During phase i small groups of people receive the trial vaccine. More than 1000 immunisations were given in the uk. The vaccine would be distributed in four phases with health care workers and vulnerable americans such as the elderly and those with underlying health conditions getting it first according to.
The first step of this extensive process involves several years of laboratory research in which scientists and. The phase ii part of the study expands the age range of people the vaccine is assessed in to include a small number of older adults and children. Internationally by november 2020 several hundred drug companies biotechnology firms university research groups and health organizations were developing over 500 potential therapies for covid19 disease in.
One typical version of phase i studies in vaccines involves an escalation study which is used in mainly medicinal research trials. The drug is introduced into a small cohort of healthy volunteers. Side effects of the vaccine are also noted and these contribute to the decision on whether to advance the candidate vaccine to a phase ii trial.
Matthew rozsa october 25 2020 1200pm utc articles about the ongoing effort to create a vaccine for the novel coronavirus often include talk of phases as if vaccine production were akin to. In phase iii the vaccine is given to thousands of people and tested for efficacy and safety.
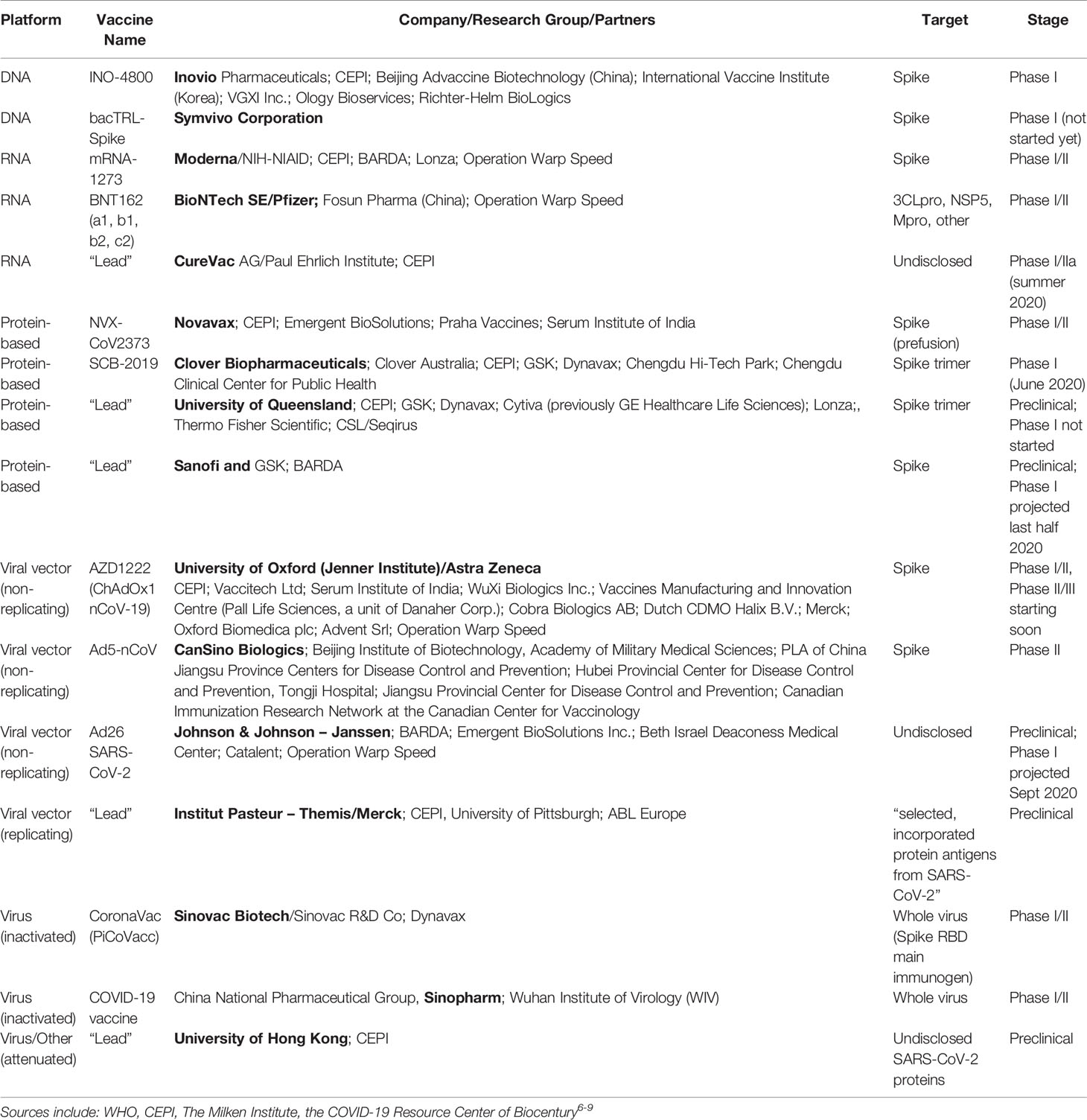
Frontiers A Snapshot Of The Global Race For Vaccines Targeting Sars Cov 2 And The Covid 19 Pandemic Pharmacology Pfizer Stock
More From Pfizer Stock
- Vaccine Schedule For Babies 2020
- Dow Jones Today Now Live Tv
- Covid Vaccine Astrazeneca Hold
- Vaccine Flue
- Down Syndrome Awareness Day 4
Incoming Search Terms:
- The Covid 19 Vaccine Development Landscape Down Syndrome Awareness Day 4,
- Covid 19 Vaccine Tracker Raps Down Syndrome Awareness Day 4,
- Trials For Three Covid 19 Vaccines Show Promise But Much More Work Still Needed Say Experts Health News Top Stories The Straits Times Down Syndrome Awareness Day 4,
- The Development And Lifespan Of A Vaccine Passport Health Down Syndrome Awareness Day 4,
- Opinion How Long Will A Vaccine Really Take The New York Times Down Syndrome Awareness Day 4,
- Vaccine Development Wur Down Syndrome Awareness Day 4,