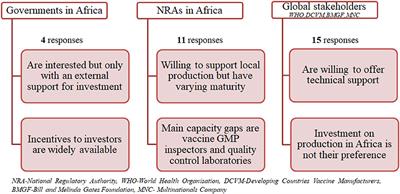
Vaccine Bulk Production, Frontiers Vaccine Production In Africa A Feasible Business Model For Capacity Building And Sustainable New Vaccine Introduction Public Health
Vaccine bulk production Indeed lately has been hunted by users around us, perhaps one of you personally. People are now accustomed to using the net in gadgets to view image and video data for inspiration, and according to the title of the article I will discuss about Vaccine Bulk Production.
- Understanding The Complexity Of Vaccine Manufacturing Sanofi
- Influenza Vaccine Manufacturing Effect Of Inactivation Splitting And Site Of Manufacturing Comparison Of Influenza Vaccine Production Processes
- Developing Countries Can Innovate And Produce Vaccines The Case Of Butantan In Brazil Intechopen
- The Engineering Challenges Of Scaling Up Uk Vaccine Manufacture The Engineer The Engineer
- Facilities Sinovac Supply Vaccines To Eliminate Human Diseases
- Incepta S Vaccine Bulk Manufacturing Facility Launched The Daily Star
Find, Read, And Discover Vaccine Bulk Production, Such Us:
- So Many Candidates So Little Time Can The World Find A Good Covid 19 Vaccine Quickly Enough Briefing The Economist
- Indonesia S Bio Farma To Get 50m Doses Of Covid 19 Bulk Vaccine Starting November Halaman All Kompas Com
- What Is The World Doing To Create A Covid 19 Vaccine Council On Foreign Relations
- Bio Farma 50 Juta Dosis Bahan Vaksin Covid 19 Sinovac Disalurkan November Maret Regional Liputan6 Com
- Coronavirus Vaccine Faces Bumpy Road From Lab To Jab Voice Of America English
If you re searching for Mmr Vaccine Uk Schedule you've arrived at the right location. We have 104 images about mmr vaccine uk schedule adding pictures, pictures, photos, wallpapers, and more. In these web page, we also provide number of graphics out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, transparent, etc.

Indonesia Teams Up With Global Manufacturers In Vaccine Hunt World The Jakarta Post Mmr Vaccine Uk Schedule
That is good news for all of us.

Mmr vaccine uk schedule. This antigen is grown in bulk collected purified and then packaged as recombinant flu vaccine. Chief operating officer coo of incepta mahbubul karim said the plant has a capacity to produce 180 million doses of vaccines a year. Recombinant vaccines are those in which genes for desired antigens of a microbe are inserted into a vectordifferent strategies are using the engineered vector eg vaccinia virus that is expressing desired antigen as a vaccine introduction of a mutation by deleting a portion of dna such that they are unlikely to revert can create an attenuated live vaccinelive attenuated vaccines can also be produced by reassortment of genomes of virulent and avirulent strains.
Us14427 for two injections once it had. Vaccines work by developing and enhancing the immunity of the body to naturally fight against serious infections and illnesses. Scale up for bulk production of vaccine against meningococcal disease.
Hence the vaccine contains nine different pora subtypes and will offer wide spread protection against meningococcal disease caused by serogroup b organisms. A vaccine bulk is an aqueous form of the purified antigens or vaccine provided in a large container from which the individual vials are filled. The purified omv bulk products of three different recombinant strains each expressing three pora subtypes are mixed and formulated to obtain the final vaccine product.
This production method does not require an egg grown vaccine virus and does not use chicken eggs at all in the production. These vaccines are then quality and potency tested by fda prior to fda approving release of the vaccine lots to the public. A cell suspension containing the cells harvested at the end of culturefermentation.
The formulated bulk prepared from one or more batches of aqueous bulk purified antigen to which adjuvant has been added present in the container from which the final containers are filled. Uk scientists start mass producing vaccines while trials are still under way. When the bulk requirement of.
More From Mmr Vaccine Uk Schedule
- Covid Vaccine Logo
- Vaccine Update For Covid 19 Russia
- Pfizer Stocks History
- Vaccine News In Hindi India
- Pfizer For Covid 19
Incoming Search Terms:
- Vaccines Development Cycle By Irish Pharmaceutical Healthcare Association Issuu Pfizer For Covid 19,
- So Many Candidates So Little Time Can The World Find A Good Covid 19 Vaccine Quickly Enough Briefing The Economist Pfizer For Covid 19,
- Bulk Manufacturing Facility Of Incepta Will Be Useful For Covid 19 Vaccine Dgda Chief The Daily Star Pfizer For Covid 19,
- Production Of Influenza Virus Vaccine Pfizer For Covid 19,
- Takeda Biological E Offer Low Cost Vaccine Combos For Asia American Pharmacy News Pfizer For Covid 19,
- Covid 19 Vaccine Makers Face Biggest Medical Manufacturing Feat In History The Globe And Mail Pfizer For Covid 19,