Quadrivalent Influenza Vaccine Sanofi Spc, The National Flu Immunisation Programme 2017 18 Training For Profes
Quadrivalent influenza vaccine sanofi spc Indeed recently is being sought by users around us, perhaps one of you. Individuals are now accustomed to using the net in gadgets to view video and image information for inspiration, and according to the name of the post I will discuss about Quadrivalent Influenza Vaccine Sanofi Spc.
- Green Cross 4 Strain Influenza Vaccine Approved By Who
- 2
- Https Www Gpni Co Uk Wp Content Uploads 2020 09 Flu Update Sides Pdf
- 2
- Http Www Wales Nhs Uk Sitesplus 888 Opendoc 338724
- Https Www Communitypharmacy Scot Nhs Uk Media 2165 Inactivated Flu Vacc Pgd 2019 20 V2 Pdf
Find, Read, And Discover Quadrivalent Influenza Vaccine Sanofi Spc, Such Us:
- Https Www Publichealth Hscni Net Sites Default Files 2018 08 Flu 20presentation 20with 20speaker 20notes 20pdf 20aq 20updated 20030818 Pdf
- Https Www Hse Ie Eng Health Immunisation Hcpinfo Conference 9ljnic2019 Pdf
- Http Sunderlandccg Nhs Uk Wp Content Uploads 2015 06 Influenza Seasonal Flu Vaccines Pdf
- Http Www Hscbusiness Hscni Net Pdf Seasonal 20influenza 20vaccination 20programme 202019 202020 Pdf
- Sanofi Ships First Flu Vaccines For 2018 2019 Season Jul 30 2018
If you are looking for Downward Dog Hand Position you've reached the right place. We have 104 images about downward dog hand position including pictures, photos, pictures, wallpapers, and much more. In these webpage, we additionally provide variety of graphics available. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.
Quadrivalent influenza vaccine split virion inactivated the summary of product characteristics spc or smpc is a specific document the wording of which has been agreed with the regulatory authority as part of the medicine approval process.
Downward dog hand position. Quadrivalent influenza vaccine split virion inactivated provides active immunisation against four influenza virus strains two a subtypes and two b types contained in the vaccine. This literature review summarizes. 90686 influenza virus vaccine quadrivalent iiv4 split virus preservative free 05 ml dosage for intramuscular use fluzone quadrivalent influenza vaccine important.
It is required before any medicine is allowed on the market in europe. Sanofi pasteur is a quadrivalent split virion influenza vaccine approved in europe in 2016 for individuals 3 years of age. Influenza virus is concentrated and purified in a linear sucrose density.
Medicare has not assigned q codes for quadrivalent influenza vaccines. Use the codes noted here as appropriate 025 ml dose taken from 5 ml multi dose vial 90687 n449281062915. A quadrivalent influenza flu vaccine is designed to protect against four different flu viruses including two influenza a viruses and two influenza b viruses.
Fluquadri for intramuscular injection is an inactivated influenza vaccine prepared from influenza viruses propagated in embryonated chicken eggs. Fluzone high dose quadrivalent is approved for use in persons 65 years of age. Fluzone quadrivalent is approved for use in persons 6 months of age and older.
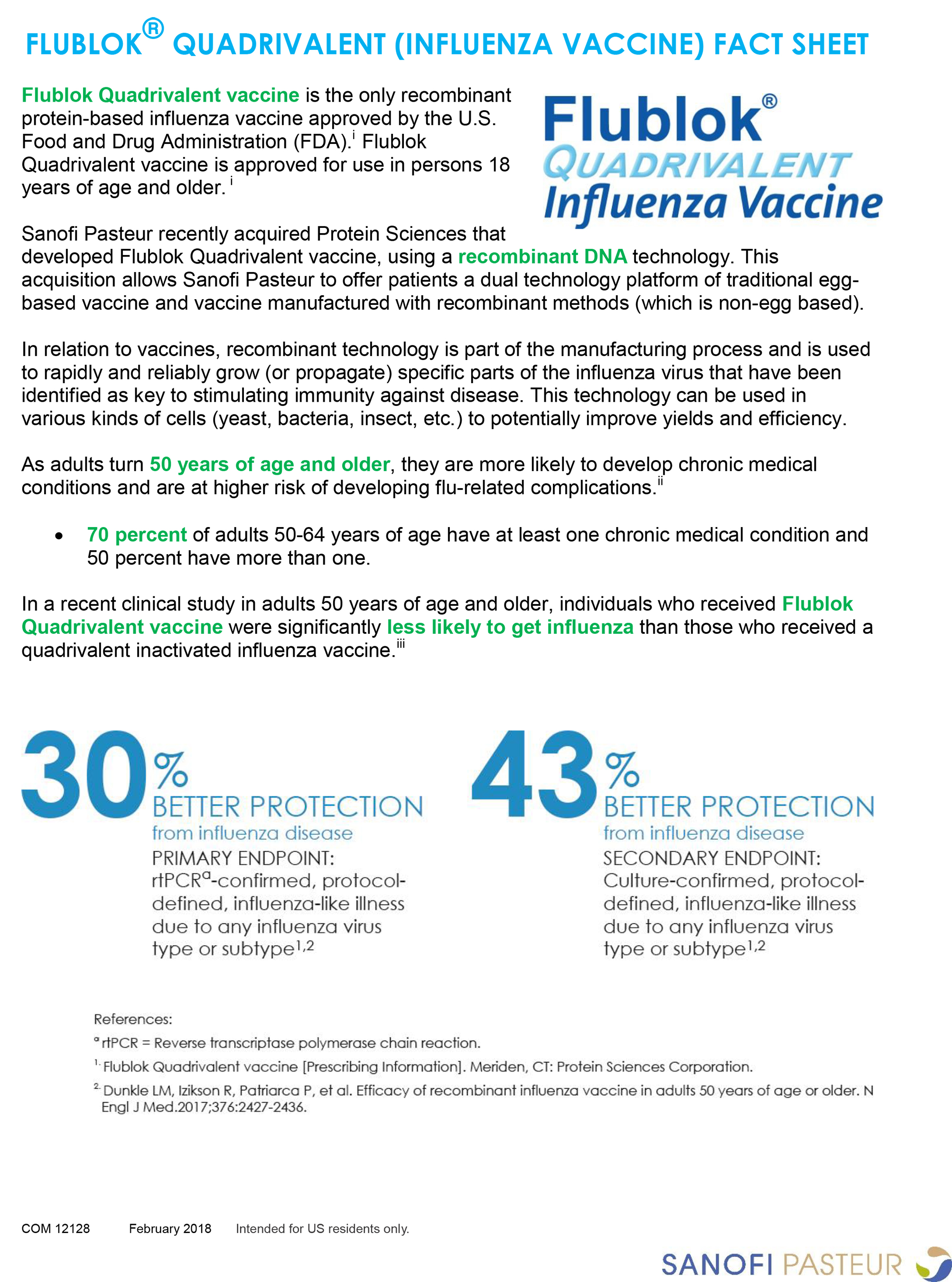
Fluzone quadrivalent flublok quadrivalent and fluzone high dose quadrivalent are vaccines indicated for active immunization for the prevention of influenza disease caused by influenza a subtype viruses and influenza type b viruses contained in the vaccine. Click here to learn more about the vaccine composition for the 2020 2021 flu season vaccines. The virus containing allantoic fluid is harvested and inactivated with formaldehyde.
Fluquadri is an inactivated quadrivalent influenza vaccine indicated for the prevention of influenza disease caused by influenza types a and b viruses contained in the vaccine. Flublok quadrivalent is approved for use in persons 18 years of age and older. Quadrivalent influenza vaccine split virion inactivated suspension for injection in pre filled syringe.
Fluquadri is approved for use in persons 6 months of age and older. Iiv4 builds on the well established record of the trivalent split virion influenza vaccine vaxigrip.
More From Downward Dog Hand Position
- Tetanus Toxoid Vaccine Schedule For Pregnant
- Pfizer Stock Options
- Dow Jones Today Right Now
- Covid Vaccine Clinical Trials Utah
- Pfizer Vaccine History
Incoming Search Terms:
- Sanofi Pasteur Ships First 2016 2017 Seasonal Influenza Vaccine Doses In United States Pfizer Vaccine History,
- Vaxigrip Tetra Nps Medicinewise Pfizer Vaccine History,
- Https Www Publichealth Hscni Net Sites Default Files 2018 08 Flu 20presentation 20with 20speaker 20notes 20pdf 20aq 20updated 20030818 Pdf Pfizer Vaccine History,
- Https Assets Publishing Service Gov Uk Government Uploads System Uploads Attachment Data File 917251 Inactivated Influenza Vaccine Information For Healthcare Practitioners 2020 To 2021 Pdf Pfizer Vaccine History,
- Sanofi Ships First Flu Vaccines For 2018 2019 Season Jul 30 2018 Pfizer Vaccine History,
- 2 Pfizer Vaccine History,