Subunit Vaccine Hepatitis B, Vaccines Springerlink
Subunit vaccine hepatitis b Indeed lately is being sought by users around us, maybe one of you. Individuals now are accustomed to using the internet in gadgets to view image and video data for inspiration, and according to the title of this post I will talk about about Subunit Vaccine Hepatitis B.
- Https Www Tri Edu Au Filething Get 45769 Hepatitis B Vaccination Fact Sheet Pdf
- Vaccines From Empirical Development To Rational Design
- Biotechnologies Applied In Biomedical Vaccines Intechopen
- Hepatitis B Vaccine
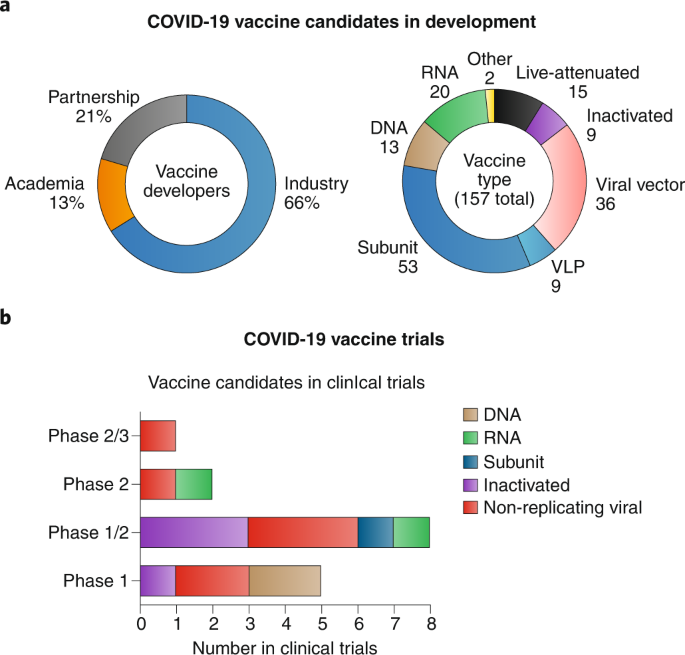
- Where Are We At With Developing A Vaccine For Coronavirus
- Solved 16 Genetically Engineered Vaccines Such As For H Chegg Com
Find, Read, And Discover Subunit Vaccine Hepatitis B, Such Us:
- Https Iubmb Onlinelibrary Wiley Com Doi Pdf 10 1042 Ba20000034
- Adapted From Project Lead The Way Making Vaccines Powerpoint Ppt Download
- بسم الله الرحمن الرحيم Immunization Immunization The Creation Of Immunity Usually Against A Particular Disease Especially Treatment As By Vaccination Ppt Download
- Module 2 Subunit Vaccines Who Vaccine Safety Basics
- Vaccine Designing
If you are looking for Vaccine Mechanism Of Action Ppt you've reached the right place. We have 100 graphics about vaccine mechanism of action ppt adding images, photos, photographs, backgrounds, and more. In such web page, we additionally provide number of images out there. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.
Hepatitis b vaccine is a vaccine that prevents hepatitis b.
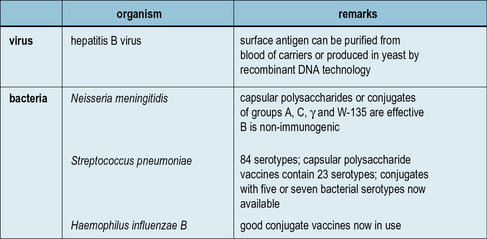
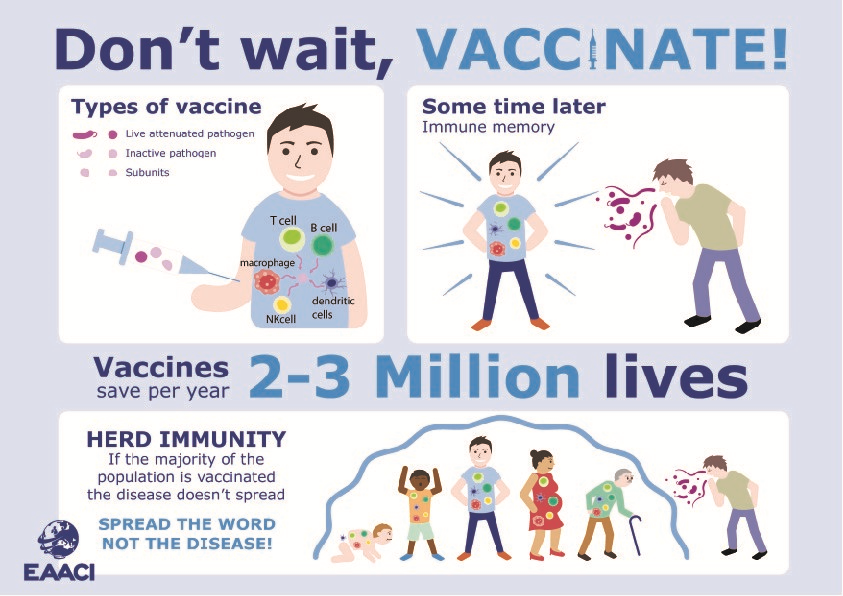
Vaccine mechanism of action ppt. This production method has been replaced by recombinant technology that can produce hbsag without requiring human plasma increasing the safety of the vaccine by excluding the risk from. It is also recommended that health care workers be vaccinated. This includes those with poor immune function such as from hivaids and those born premature.
The vaccines differ only in the concentration of the hbsag antigen and the nature of aluminum adjuvants. Recombivax contains aluminum hydrophosphate sulphate. Making vaccines subunit vaccine.
Following stringent inactivation procedures the purified hbsag was formulated with an adjuvant and for multi dose vials thiomersal was added as a preservative. The 22 nm spherical form of hepatitis b surface antigen was purified from the serum or plasma of chronic carriers of the antigen. Earlier vaccine products were produced using purified plasma of infected individuals.
Hepatitis b subunit vaccine. Hepatitis b subunit vaccine. Just like the highly successful subunit vaccines the recombinant vector produced antigen will be of little to no risk to the patient.
Step 1 use the tweezers to pull out a segment of dna from the hepatitis b virus. In healthy people routine immunization results in more than 95 of. Guidelines for the preparation of the 22 nm spherical particles and their separated polypeptides derived from the plasma of asymptomatic human carriers were suggested by the who expert committee on viral hepatitis in 1977 and the proposed requirements for the 22 nm.
The first dose is recommended within 24 hours of birth with either two or three more doses given after that. 828832 abstract the 22 nm spherical form of hepatitis b surface antigen was purified from the serum or plasma of chronic carriers of the antigen. A portion of the hepatitis b virus gene coding for hbsag is cloned into yeast and the vaccine for hepatitis b is produced from cultures of this recombinant yeast strain according to.
Recombivax and engerixb are licensed for both adult and pediatric populations. A subunit vaccine makes use of just a small portion of a pathogen. This is the type of vaccine currently in use for hepatitis b and it is experimentally popular being used to try to develop new vaccines for difficult to vaccinate against viruses such as ebolavirus and hiv.
A preliminary report of safety and efficacy tests in chimpanzees. Both are recombinant subunit vaccines containing hbsag protein. Purcell rh gerin jl.
Hepatitis b vaccines are composed of the hepatitis b virus surface antigen hbsag a protein produced by hepatitis b virus. Antigens of subtypes ayw and adr were individually prepared by isopycnic banding in cesium chloride. Authors r h purcell j l gerin.
Recombivax hb hepatitis b vaccine recombinant is a non infectious subunit viral vaccine derived from hepatitis b surface antigen hbsag produced in yeast cells. Hepatitis b surface antigen in the form of 22 nm spherical particles and tubular forms is excess virus coat protein.
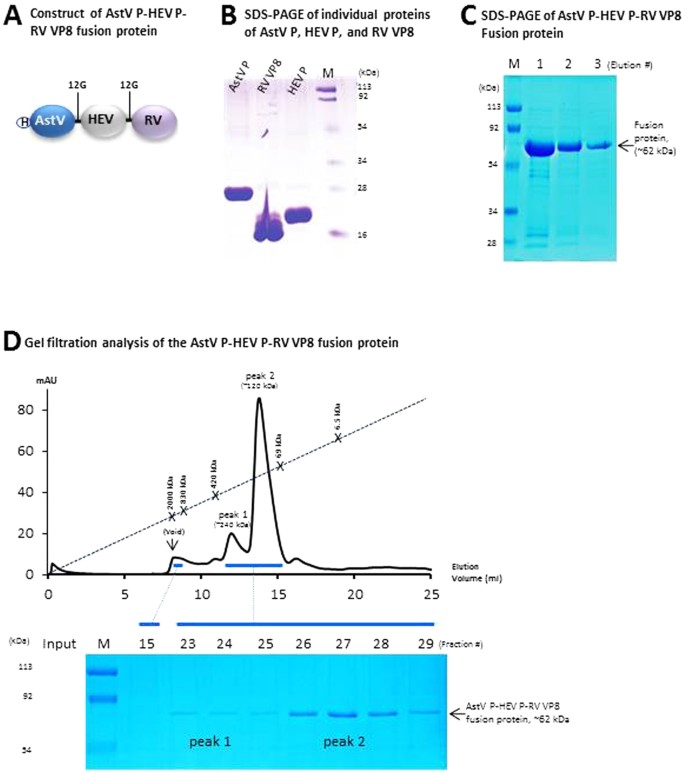
Development And Evaluation Of Two Subunit Vaccine Candidates Containing Antigens Of Hepatitis E Virus Rotavirus And Astrovirus Scientific Reports Vaccine Mechanism Of Action Ppt
More From Vaccine Mechanism Of Action Ppt
- Hepatitis B Vaccine Schedule For Adults In Uae
- 15 Years Vaccine Name
- Pfizer Vaccine Phase 2 Results
- Dpt Vaccine Images
- Pfizer Stock Split Date
Incoming Search Terms:
- Prevention Of Hepatitis B With The Hepatitis B Vaccine Nejm Pfizer Stock Split Date,
- Hepatitis B Vaccine Pfizer Stock Split Date,
- Effect Of Vaccination Of Humans With A Subunit Hepatitis B Vaccine On Download Scientific Diagram Pfizer Stock Split Date,
- So Many Candidates So Little Time Can The World Find A Good Covid 19 Vaccine Quickly Enough Briefing The Economist Pfizer Stock Split Date,
- Polymers For Subunit Vaccine Delivery Sciencedirect Pfizer Stock Split Date,
- Measles And Vaccines Vaccine Intro Youtube Pfizer Stock Split Date,