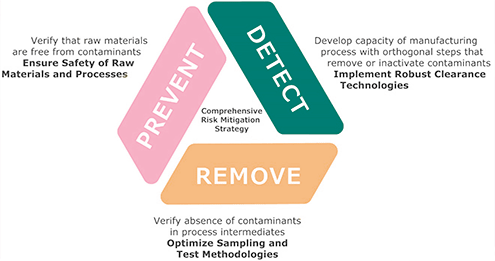
Viral Vector Vaccine Manufacturing Process, Is The World Ready To Produce A Billion Doses Of A Covid 19 Vaccine Imperial News Imperial College London
Viral vector vaccine manufacturing process Indeed lately is being sought by users around us, perhaps one of you. People are now accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this post I will talk about about Viral Vector Vaccine Manufacturing Process.
- Aav Manufacturing Herpes Assisted Expansion Bioprocess Internationalbioprocess International
- Scalable Purification Of Adenovirus Serotype 5 Viral Vector Using Ion Exchange Mustang Q Membranes
- Vaccine Clarification With Filter Based Methods And Vector Clarificationbioprocess International
- Production Of A Chikungunya Vaccine Using A Cho Cell And Attenuated Viral Based Platform Technology Molecular Therapy
- A Visual Aid To Viral Infection And Vaccine Production Charlotte Lozier Institute
- Covid 19 Halix Ready For Vaccine Production European Biotechnology
Find, Read, And Discover Viral Vector Vaccine Manufacturing Process, Such Us:
- Why A Coronavirus Vaccine Takes So Long To Develop Business Insider
- Individualized Nhl Vaccine Manufacturing Process Overview Download Scientific Diagram
- Vaccine Wikipedia
- Is The World Ready To Produce A Billion Doses Of A Covid 19 Vaccine Imperial News Imperial College London
- Towards Effective Covid 19 Vaccines Updates Perspectives And Challenges Review
If you re searching for Hepatitis B Vaccine Schedule For Adults you've reached the perfect location. We have 104 graphics about hepatitis b vaccine schedule for adults adding pictures, photos, pictures, wallpapers, and more. In such web page, we additionally have variety of images out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.
Delivery of genes or other genetic material by a vector is termed transduction and the.
Hepatitis b vaccine schedule for adults. Complex multistep processes are required in both cases but the low virus titers in batch cultures and the temperature sensitivity of the virus particles limit the production scale. The use of process analytical technologies in viral vector applications enables process controls and monitoring to achieve a higher viral vector. Viral vector vaccines combine many of the positive qualities of dna vaccines with those of live attenuated vaccines.
Cobra has been a manufacturer of gmp viral vector products for gene therapies and viral vaccines since 2002 and more recently immuno oncology therapies. Cell culture from research and process development to large scale manufacturing. To meet market needs for scalable and cost effective manufacturing of viral vector systems scientists at cytiva have developed a process for adenovirus production from upstream cell culture to downstream purification using modern tools and technologies.
The developed process is easily scaled and compatible with both single use and steamable. Viral vector vaccine design. Viral vectors are tools commonly used by molecular biologists to deliver genetic material into cellsthis process can be performed inside a living organism or in cell culture viruses have evolved specialized molecular mechanisms to efficiently transport their genomes inside the cells they infect.
Viral vectors are promising tools for the development of novel vaccines and vaccination approaches. Our team provides gmp manufacture of viral vector products following good manufacturing practices of production and testing for all viral manufacturing programs to ensure quality products for our customers preclinical and clinical trials. Robust viral vector propagation in vaccine development relies on scalability from process development to production scale manufacturing while ensuring containment in a biosafety level 2 environment.
The industrial scale manufacturing of viruses or virus like particles in cell culture is necessary for gene therapy and the treatment of cancer with oncolytic viruses. Gardasil an anti human papilloma virus vaccine that is very effective in preventing cervical cancer the current hepatitis b vaccine is also this. To meet commercial and regulatory requirements.
This process induces the vector to produce an antigen which is then purified the purified antigen when combined with an adjuvant results in a safe and very effective vaccine example. 1 like dna vaccines viral vector vaccines carry dna into a host cell for production of antigenic proteins that can be tailored to stimulate a range of immune responses including antibody t helper cell cd4 t cell and cytotoxic t lymphocyte ctl cd8 t cell. Our peptone supplements chemically defined and serum free media for virus and vaccine production deliver cell growth and virus production equivalent or superior to serum supplemented systems while also maximizing consistency and reliability and simplifying downstream purification.
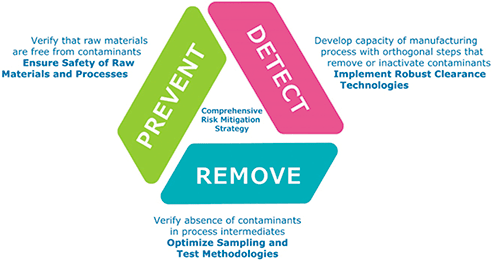
Ensuring Viral Safety Of Viral Vaccines And Vectors Bioprocess Development Forum Hepatitis B Vaccine Schedule For Adults
More From Hepatitis B Vaccine Schedule For Adults
- Covid Vaccine News Russian
- Vaccine Manufacturing Vaccine Development Process
- Covid Vaccine Human Trials Results
- Coronavirus Vaccine In India Update
- 20 Week Down Syndrome Baby Ultrasound Picture
Incoming Search Terms:
- Vaccine Clarification With Filter Based Methods And Vector Clarificationbioprocess International 20 Week Down Syndrome Baby Ultrasound Picture,
- Covid 19 Halix Ready For Vaccine Production European Biotechnology 20 Week Down Syndrome Baby Ultrasound Picture,
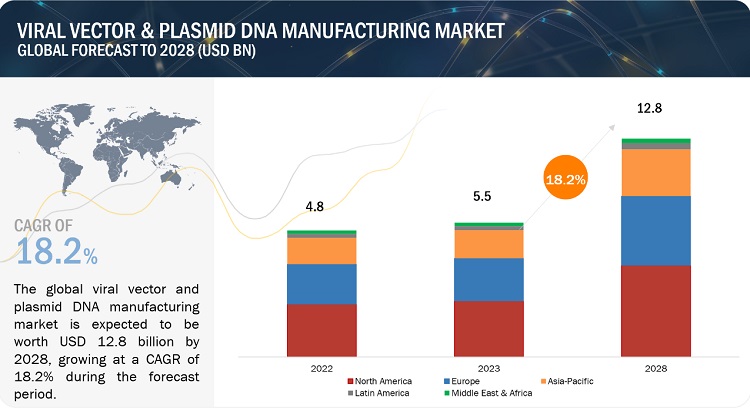
- Viral Vectors Plasmid Dna Manufacturing Market Report 2027 20 Week Down Syndrome Baby Ultrasound Picture,
- A Visual Aid To Viral Infection And Vaccine Production Charlotte Lozier Institute 20 Week Down Syndrome Baby Ultrasound Picture,
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsclz4a2lmzxiw5wzdhdfqx4noaz6x6l4izef0e2mnczga8rinl Usqp Cau 20 Week Down Syndrome Baby Ultrasound Picture,
- Scalable Viral Vector Purification For Gene Therapy Appraisal Of Downstream Processing Approachesbioprocess International 20 Week Down Syndrome Baby Ultrasound Picture,