Vaccine Trial Phases Timeline, Coronavirus Vaccine Faces Bumpy Road From Lab To Jab Voice Of America English
Vaccine trial phases timeline Indeed recently is being hunted by consumers around us, maybe one of you personally. People now are accustomed to using the net in gadgets to view image and video data for inspiration, and according to the name of this article I will talk about about Vaccine Trial Phases Timeline.
- Timeline Shows 3 Paths To Covid 19 Treatment And Prevention Infographic
- Vaccine Development Chart Aaf
- Overview Of The Respiratory Syncytial Virus Vaccine Candidate Pipeline In Canada Ccdr 46 4 Canada Ca
- Coronavirus Vaccine How Soon Will We Have One World Economic Forum
- Ube9llt Qsifym
- Coronavirus How Soon Can We Expect A Working Vaccine Bbc News
Find, Read, And Discover Vaccine Trial Phases Timeline, Such Us:
- Why A Coronavirus Vaccine Takes Over A Year To Produce And Why That Is Incredibly Fast World Economic Forum
- Developing Covid 19 Vaccines At Pandemic Speed Nejm
- Coronavirus Researchers Compete To Enroll Subjects For Vaccine Tests Wsj
- How Long Does It Take To Develop A Vaccine World Economic Forum
- Covid 19 Vaccine Development Across The Globe Clinical Trials Arena
If you are searching for Pfizer Covid Vaccine Open Letter you've come to the perfect place. We ve got 100 images about pfizer covid vaccine open letter adding pictures, photos, pictures, wallpapers, and more. In these page, we also provide variety of graphics available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, transparent, etc.
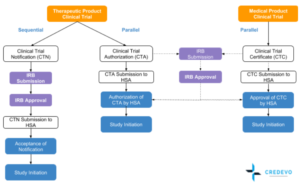
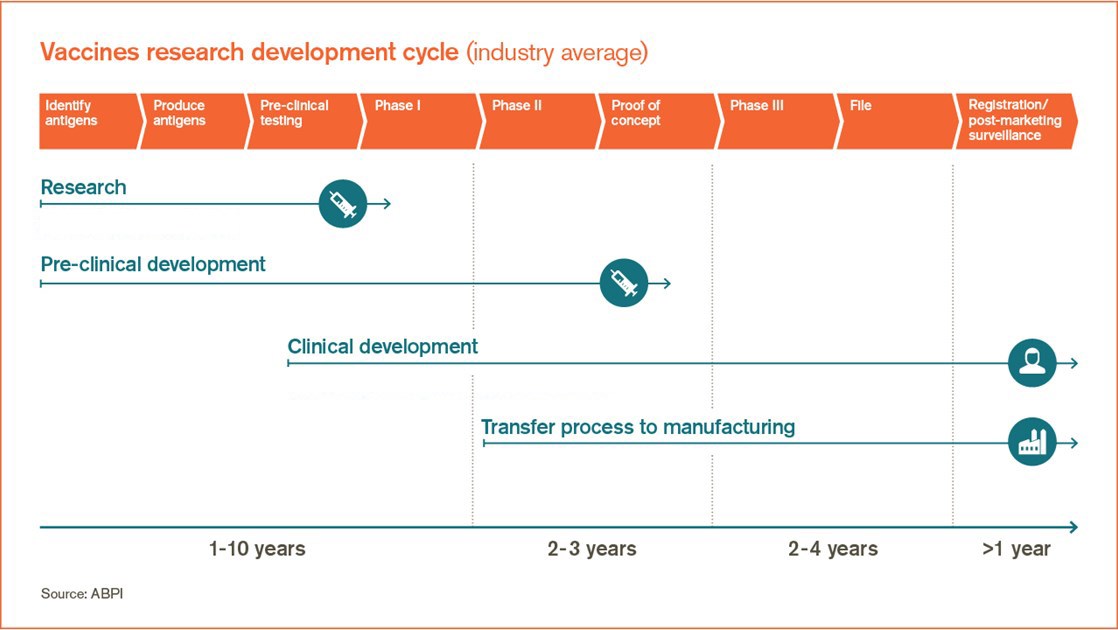
A vaccine candidate drug is first identified through preclinical evaluations that could involve high throughput screening and selecting the proper antigen to invoke an immune response.

Pfizer covid vaccine open letter. Vaccine clinical development follows the same general pathway as for drugs and other biologics. A vaccine trial is a clinical trial that aims at establishing the safety and efficacy of a vaccine prior to it being licensed. During phase i small groups of people receive the trial vaccine.
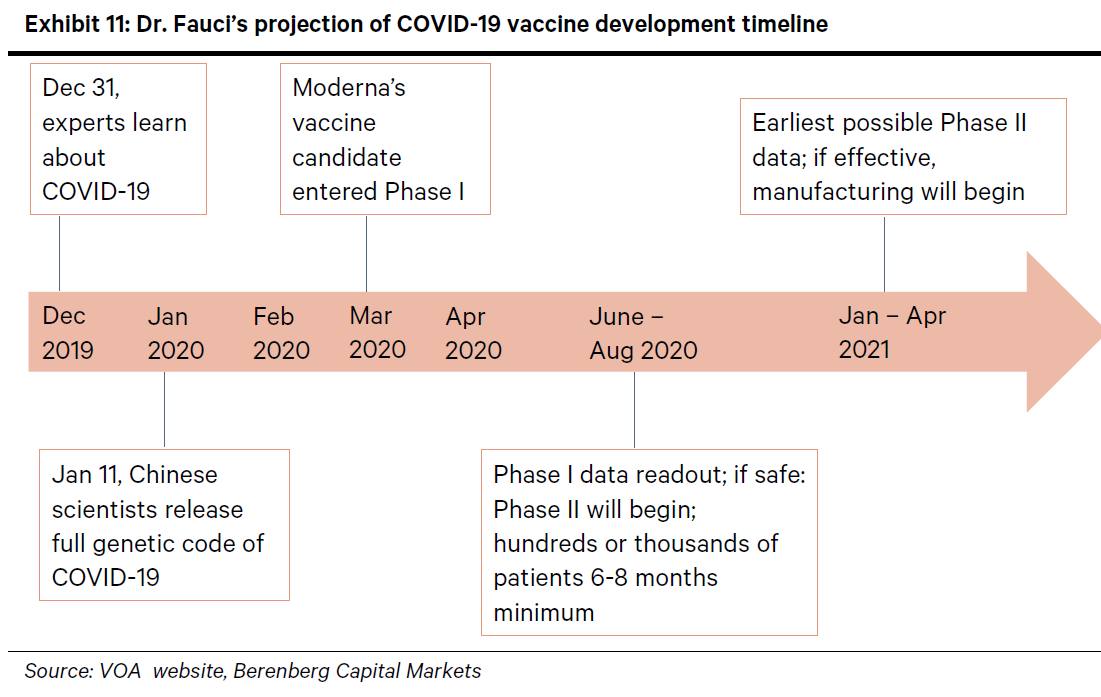
Moderna the trial is designed for people to receive a second shot either three or four weeks later. These are some of the vaccines candidates making the most progress so far. Phase 1 testing marks the first time the vaccine is tested in a small group of adults usually between 20 to 80 people to evaluate its safety and measure the immune response it generates.
But tuesday astrazeneca one of three companies already in phase 3 trials revealed it had halted its covid 19 trials worldwide. Sii the worlds largest. In phase ii the clinical study is expanded and vaccine is given to people who have characteristics such as age and physical health similar to those for whom the new vaccine is intended.
In phase iii the vaccine is given to thousands of people and tested for efficacy and safety. A vaccine trial can go bad even if the vaccine is good. If successful the vaccine could.
Sanofi a french biopharmaceutical company expects to begin clinical trials late this year for a covid 19 vaccine that it repurposed from work on a sars vaccine. The vaccine began phase 23 trials in england and india where its known as covishield. In addition astrazeneca launched phase 3 trials in brazil south africa and the united states.
Phase 2a studies aim to determine the most effective dose and expand the safety experience with the vaccine.

Estimating The Cost Of Vaccine Development Against Epidemic Infectious Diseases A Cost Minimisation Study The Lancet Global Health Pfizer Covid Vaccine Open Letter
More From Pfizer Covid Vaccine Open Letter
- Dow Jones Chart 1929 To 1933
- Pfizer Covid Vaccine Refrigerator
- How Vaccine Works Gif
- Vaccine Production Capacity Per Day
- Eva Longoria Short Hairstyles
Incoming Search Terms:
- Frontiers A Snapshot Of The Global Race For Vaccines Targeting Sars Cov 2 And The Covid 19 Pandemic Pharmacology Eva Longoria Short Hairstyles,
- Why A Coronavirus Vaccine Takes Over A Year To Produce And Why That Is Incredibly Fast World Economic Forum Eva Longoria Short Hairstyles,
- Coronavirus Vaccine Faces Bumpy Road From Lab To Jab Voice Of America English Eva Longoria Short Hairstyles,
- Developing Covid 19 Vaccines At Pandemic Speed Nejm Eva Longoria Short Hairstyles,
- U K Starts Oxford Coronavirus Vaccine Trial As Germany Green Lights Biontech And Pfizer Eva Longoria Short Hairstyles,
- Will Big Tobacco Save Us From The Coronavirus Ft Alphaville Eva Longoria Short Hairstyles,
/cdn.vox-cdn.com/uploads/chorus_asset/file/19923279/Screen_Shot_2020_04_24_at_4.08.10_PM.png)