Vaccine Research And Development, Rsv Vaccine Research And Development Technology Roadmap Priority Activities For Development Testing Licensure And Global Use Of Rsv Vaccines With A Specific Focus On The Medical Need For Young Children In Low
Vaccine research and development Indeed recently is being sought by users around us, perhaps one of you personally. People are now accustomed to using the internet in gadgets to see image and video data for inspiration, and according to the title of this article I will discuss about Vaccine Research And Development.
- Covid 19 Vaccine Research And Development Hillnotes
- Covid 19 Vaccine Research And Development Landscape July 2020 L E K Consulting
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrqygduldesofqayam Kf88wsgfwurr1v 5u0bjjkmysz0cssew Usqp Cau
- Coronavirus Covid 19 Medical Test Vaccine Research And Development Concept Stock Image Image Of Laboratory Corona 182425835
- Memo To Dfat Fund Vaccines Devpolicy Blog From The Development Policy Centre
- The Mosaic Of Coronavirus Vaccine Development Systemic Failures In Vaccine Innovation Jia Sipa
Find, Read, And Discover Vaccine Research And Development, Such Us:
- Vaccine Research And Development Ifpma
- Pdf Update On Research And Development Pipeline Tuberculosis Vaccines Semantic Scholar
- B Cell Sequencing For Rapid Vaccine Research And Development
- Enviralmental Viral Vaccine Research And Development An Alumni Interview With Dr Anh Tran Immpress Magazine
- Sars Cov 2 Vaccine Research And Development Conventional Vaccines And Biomimetic Nanotechnology Strategies Sciencedirect
If you are looking for Covid Vaccine Date India you've arrived at the right place. We ve got 104 graphics about covid vaccine date india including pictures, photos, pictures, backgrounds, and much more. In these page, we additionally provide variety of graphics out there. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.
Pfizer has a rich history in vaccine research and development.

Covid vaccine date india. Vaccine development involves the process of taking a new antigen or immunogen identified in the research process and developing this substance into a final vaccine that can be evaluated through preclinical and clinical studies to determine the safety and efficacy of the resultant vaccine. Development of new vaccines. Ivr activities align with the strategic objective 6 of the global vaccine action plan country regional and global research and development innovations maximize the benefits of immunization and with the fifth goal of the decade of vaccines.
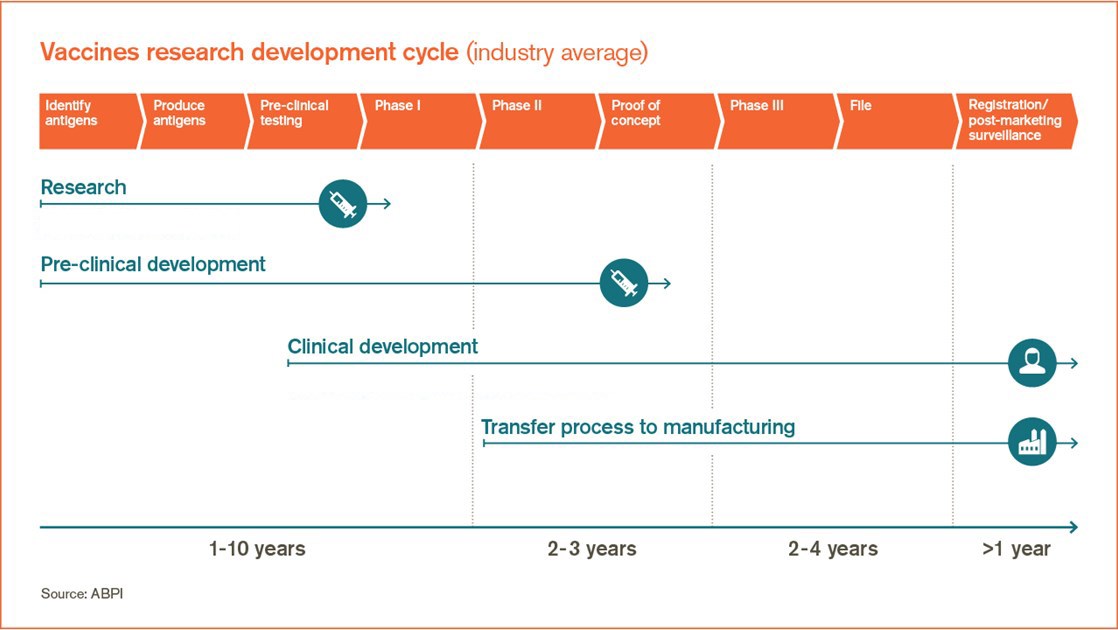
A database was actively compiled to include all vaccine projects in development from 1998 to 2009 in the pre clinical development phase clinical trials phase i ii and iii up to market registration. The general stages of the development cycle of a vaccine are. Vaccines are one of the greatest public health advancements of all time resulting in the control elimination or near elimination of numerous infectious diseases that were once pervasive and often fatal.
The average vaccine taken from the preclinical phase requires a development timeline of 1071 years and has a market entry probability of 6. While the antigen changes in the flu vaccine every year the manufacturing process remains the same and is founded on. Rsv vaccine research and development technology roadmap.
Food and drug administration fda seasonal flu vaccines are developed on an annual basis. Vaccine research development the field deals collectively with various kinds of vaccines their synthesis therapeutic effects and clinical developments. This page leads to other pages that describe vaccine development and testing such as basic research clinical studies side effects and adverse reactions vaccines of the future and the vaccine product approval process.
Unlike other vaccines which typically take 10 to 15 years of research development and testing before being approved by the us. The field entirely covers all the advancements developments of vaccines in every field of vaccination. Priority activities for development testing licensure and global use of rsv vaccines with a specific focus on the medical need for young children in low and middle income countries 11 august 2017.
More From Covid Vaccine Date India
- Vaccine Of Covid 19 Update
- Flu Jab Live Vaccine 2020
- Hepatitis B Vaccine Schedule For Newborn
- Stocks Meme Png
- Apple Stock Price History 10 Years
Incoming Search Terms:
- Imperial Network For Vaccine Research Research Groups Imperial College London Apple Stock Price History 10 Years,
- Covid 19 Vaccine Development And A Potential Nanomaterial Path Forward Nature Nanotechnology Apple Stock Price History 10 Years,
- Southeast Republican House Members Work To Secure 10 Million For Covid 19 Vaccine Research And Development Lower Bucks Times Apple Stock Price History 10 Years,
- Innovation Partnership For A Roadmap On Vaccines In Europe Iprove A Vision For The Vaccines Of Tomorrow Sciencedirect Apple Stock Price History 10 Years,
- Hiv Vaccine And Biomedical Prevention Research U S Agency For International Development Apple Stock Price History 10 Years,
- B Cell Sequencing For Rapid Vaccine Research And Development Apple Stock Price History 10 Years,