Vaccine Phase 3 Length, Uae And China Launch Phase 3 Clinical Trial In Humans For Covid 19 Vaccine Cnn
Vaccine phase 3 length Indeed lately has been sought by consumers around us, maybe one of you. Individuals now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will talk about about Vaccine Phase 3 Length.
- How Long Does It Take To Develop A Vaccine World Economic Forum
- On Pins And Needles Will Covid 19 Vaccines Save The World Mckinsey
- First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
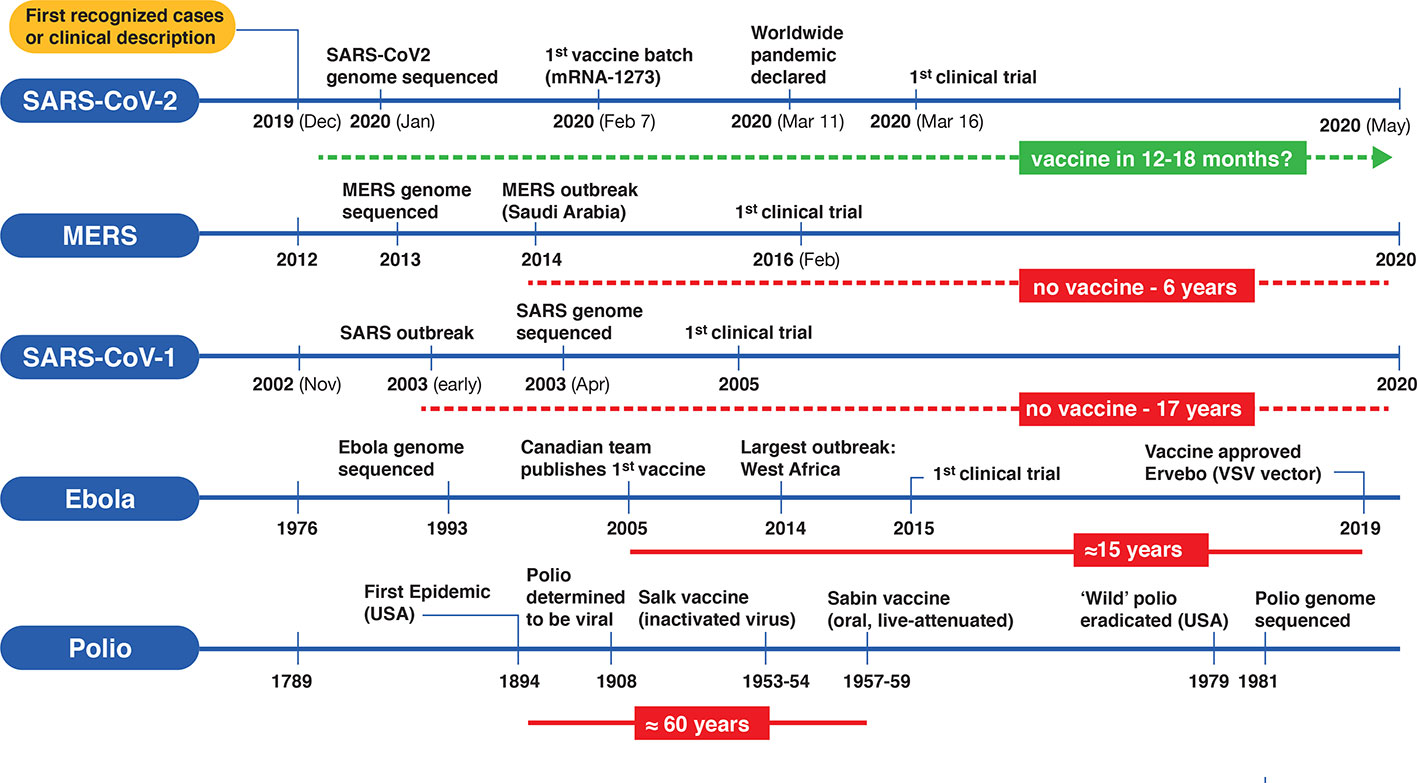
- What History Tells Us About Vaccine Timetables Charles River Laboratories
- How Long Does It Take To Develop A Vaccine World Economic Forum
- Pandemic Influenza Vaccine Manufacturing Process And Timeline
Find, Read, And Discover Vaccine Phase 3 Length, Such Us:
- Moderna S Covid 19 Vaccine Enters Final Testing Phase Time
- Xim6ucijflbzim
- Moderna Coronavirus Vaccine Starts Phase 3 Trial What To Expect Business Insider
- First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
- Safety And Immunogenicity Of The Tetravalent Live Attenuated Dengue Vaccine Butantan Dv In Adults In Brazil A Two Step Double Blind Randomised Placebo Controlled Phase 2 Trial The Lancet Infectious Diseases
If you re searching for Vaccine Ingredients Chart you've arrived at the perfect place. We ve got 100 graphics about vaccine ingredients chart including pictures, photos, photographs, wallpapers, and more. In these page, we also have number of graphics out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
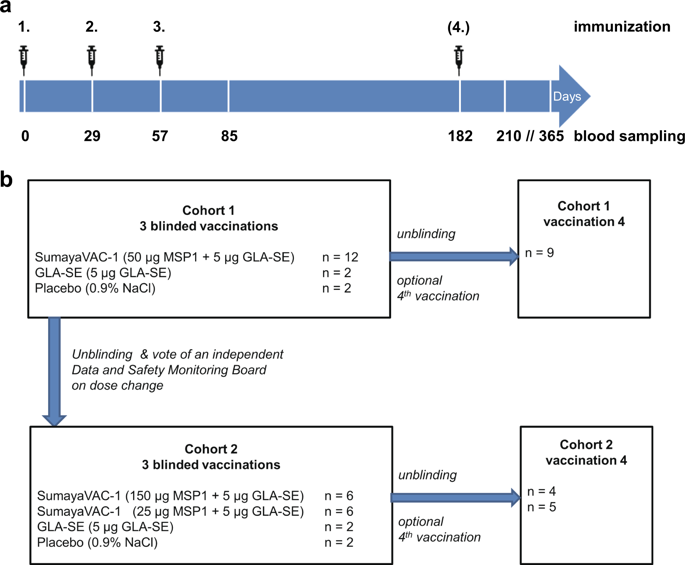
Immunization With Full Length Plasmodium Falciparum Merozoite Surface Protein 1 Is Safe And Elicits Functional Cytophilic Antibodies In A Randomized First In Human Trial Npj Vaccines X Mol Vaccine Ingredients Chart
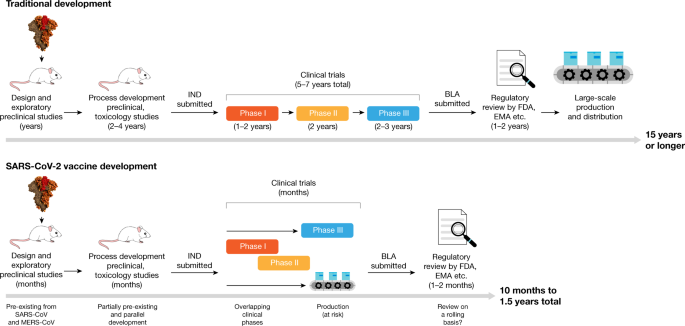
A phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 covid 19 in adults has begun.
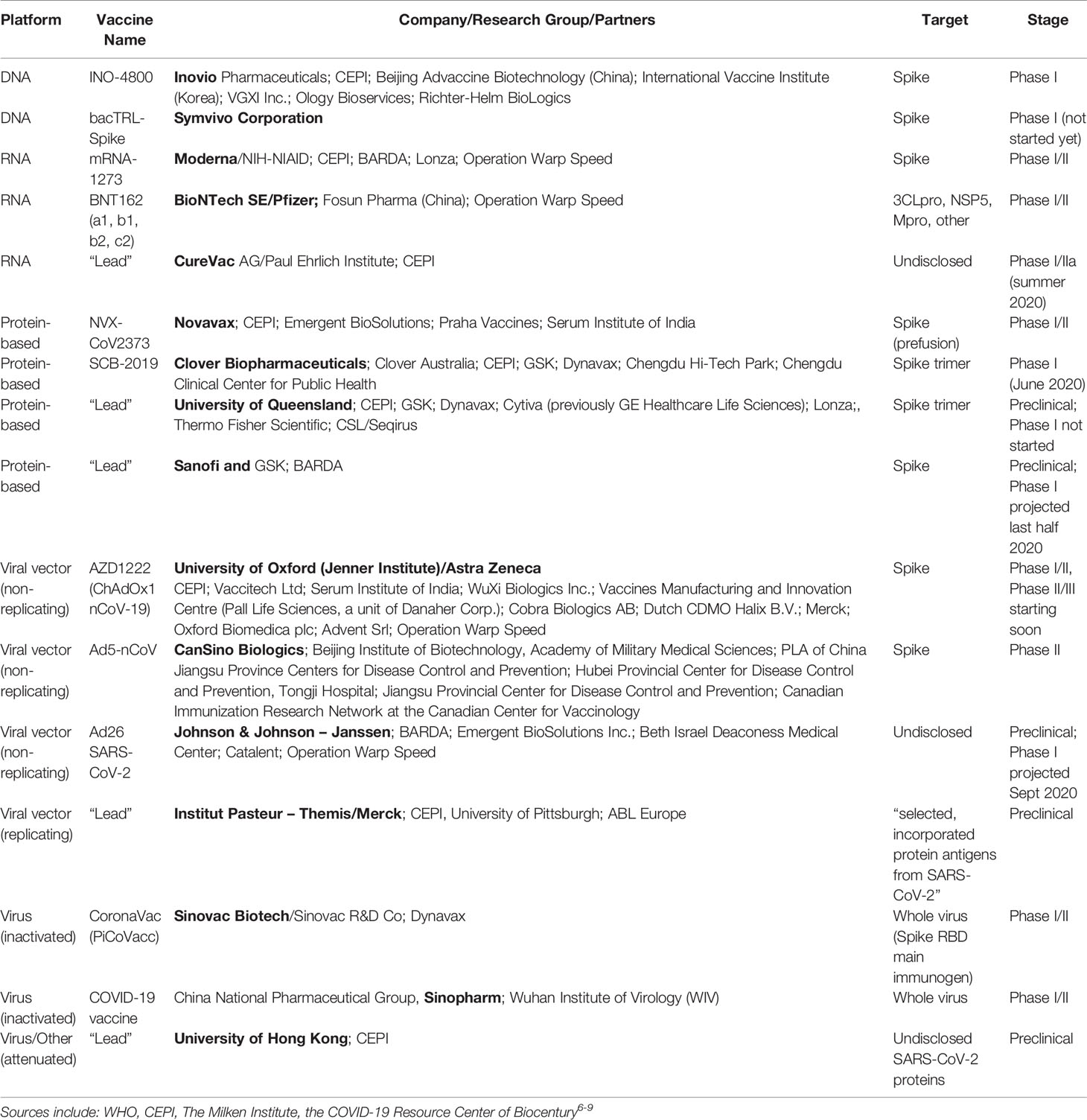
Vaccine ingredients chart. This trial will include 30000 health adults in the us and is expected to expand to 120 sites globally. In phase ii the clinical study is expanded and vaccine is given to people who have characteristics such as age and physical health similar to those for whom the new vaccine is intended. Phase 3 trials test a vaccines safety and efficacy on a larger number of people across multiple locations.
Moderna has manufactured the required vaccine quantity for phase 3 and says it remains on track to deliver between 500 million and 1 billion doses per year at 100 mg. 20 of healthy adults to test the properties of a vaccine tolerability and if appropriate clinical laboratory and pharmacological parameters. The vaccine known as mrna 1273 was co developed by the cambridge massachusetts based biotechnology company moderna inc and the national institute of allergy and infectious diseases.
Volunteer participating in phase 3 trial of the sinovac covid 19 vaccine in padjadjaran university bandung west java indonesia. A vaccine trial is a clinical trial that aims at establishing the safety and efficacy of a vaccine prior to it being licensed. The vaccine is expected to enter phase 3 testing next week.
In phase i clinical studies initial testing of a vaccine is carried out in small numbers eg. Clinical development is a three phase process. This phase of the trial is expected to involve 30000 volunteers and will test whether the vaccine protects people against the coronavirus.
The vaccine currently in phase iii of its trials which was considered to be the first one to get launched for the public in 2020 will be most likely available for public deployment by 2021. A fourth covid 19 vaccine candidate has gone into the final stage of clinical trials in the us with johnson johnson announcing the start of its phase 3 trial wednesday.
More From Vaccine Ingredients Chart
- Downton Abbey Cast
- Vaccine Research And Development Journal
- Downton Abbey Costumes For Sale
- China Coronavirus Vaccine News Update
- Pfizer Covid Vaccine Protocol
Incoming Search Terms:
- Xim6ucijflbzim Pfizer Covid Vaccine Protocol,
- Estimating The Cost Of Vaccine Development Against Epidemic Infectious Diseases A Cost Minimisation Study The Lancet Global Health Pfizer Covid Vaccine Protocol,
- Covid 19 Vaccines Breaking Record Times To First In Human Trials Npj Vaccines Pfizer Covid Vaccine Protocol,
- Safety And Immunogenicity Of The Tetravalent Live Attenuated Dengue Vaccine Butantan Dv In Adults In Brazil A Two Step Double Blind Randomised Placebo Controlled Phase 2 Trial The Lancet Infectious Diseases Pfizer Covid Vaccine Protocol,
- Covid 19 Vaccines A Race Against Time In The Middle Of Death And Devastation Journal Of Clinical And Experimental Hepatology Pfizer Covid Vaccine Protocol,
- Frontiers A Snapshot Of The Global Race For Vaccines Targeting Sars Cov 2 And The Covid 19 Pandemic Pharmacology Pfizer Covid Vaccine Protocol,