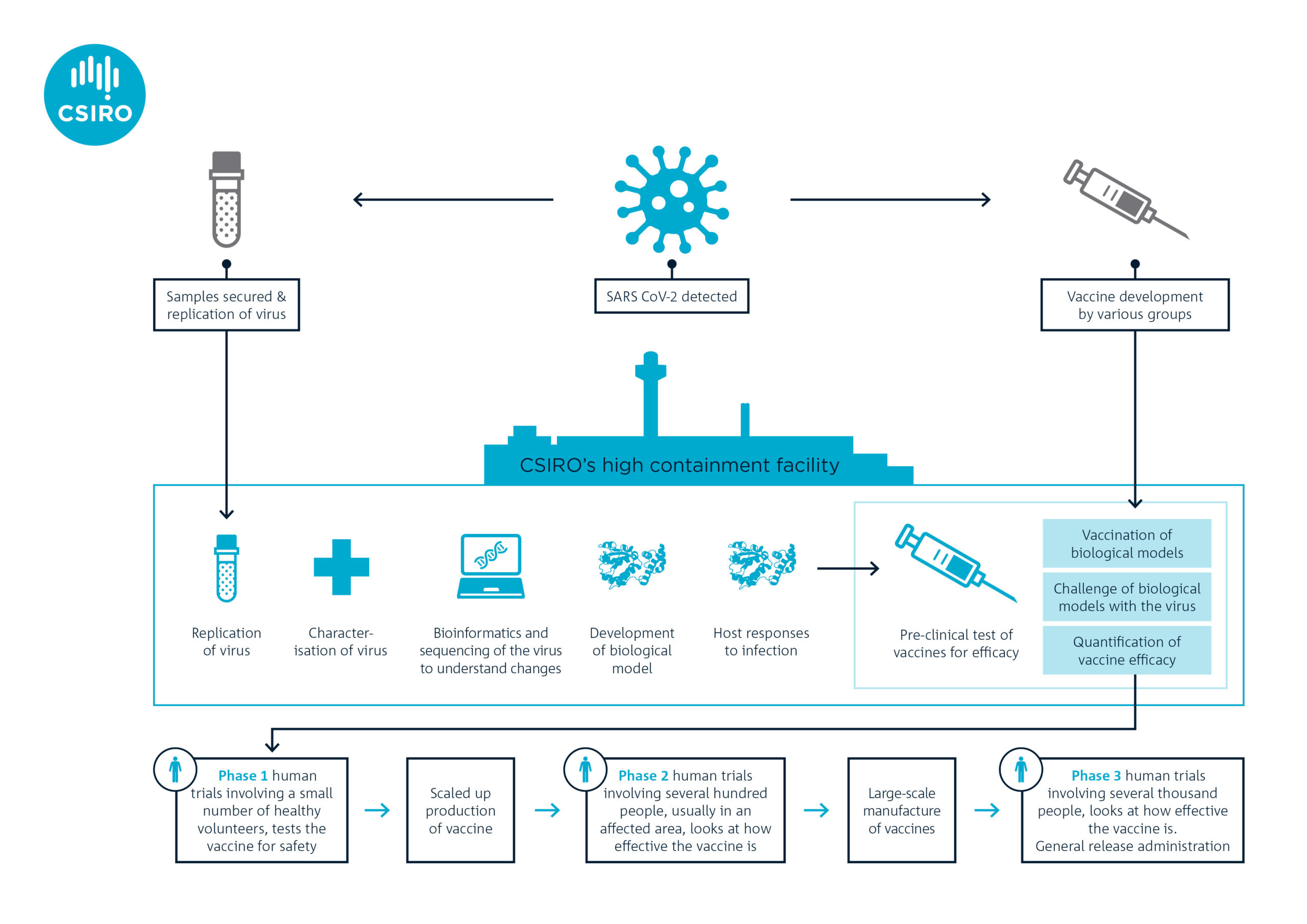
Vaccine Manufacturing Process Diagram, Emerging Technologies For Low Cost Rapid Vaccine Manufacture Kis 2019 Biotechnology Journal Wiley Online Library
Vaccine manufacturing process diagram Indeed recently has been hunted by users around us, perhaps one of you. Individuals now are accustomed to using the net in gadgets to view image and video data for inspiration, and according to the name of the post I will talk about about Vaccine Manufacturing Process Diagram.
- Video An Infectious Disease Expert Explains The Results From Moderna S Latest Vaccine Trials
- How Close Is A Coronavirus Vaccine Free To Read Financial Times
- Covid 19 Halix Ready For Vaccine Production European Biotechnology
- Risk Based Bioengineering Strategies For Reliable Bacterial Vaccine Production Sciencedirect
- Flow Chart For Vaccine Production Diagram Png Image With Transparent Background Toppng
- Plos One Influenza Vaccine Manufacturing Effect Of Inactivation Splitting And Site Of Manufacturing Comparison Of Influenza Vaccine Production Processes
Find, Read, And Discover Vaccine Manufacturing Process Diagram, Such Us:
- Optimizing The Utilization Of Aluminum Adjuvants In Vaccines You Might Just Get What You Want Npj Vaccines
- Vaccine Development Services Charles River
- Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International
- Vaccines Free Full Text Efforts To Improve The Seasonal Influenza Vaccine Html
- The Art Of Partnerships For Vaccines Sciencedirect
If you are searching for Vaccine Phase 1 2 3 you've reached the ideal location. We ve got 104 graphics about vaccine phase 1 2 3 adding images, pictures, photos, backgrounds, and more. In such webpage, we also have number of images available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.
Https Www Bio Fiocruz Br En Images Stories Pdfs Mpti 2013 Selecao Vaccine Process Technology Pdf Vaccine Phase 1 2 3
Ples that any procedure process equip ment material activity or system leads to the results expected.

Vaccine phase 1 2 3. The nal vaccine manufacturing process and must embody the. From laboratory method and phase i trials. Until recently this production process also began with egg grown cvvs per fda regulations.
Stages of vaccine production vaccine production has several stages. This large scale production is often a challenge. Antonia in gene therapy of cancer third edition 2014.
There also is a cell based production process for flu vaccines that was approved by fda in 2012. Methods thatensure product quality and standards for the. The discovery that dcs can be generated from monocytes or cd34 precursors permitted harvesting of these rare cells in large numbers for.
In phase iii. Process of vaccine manufacture has the following steps. There should be complete validation of manufacturing processes to ensure the continuous conformity of vaccine batches to required standards ec 1990a.
A general flow diagram of a purification train in the vaccine production process paul k ng. The first step in ex vivo vaccine production is the generation of an enriched population of mature dendritic cells for vaccines. Vaccine manufacturers apply to the fda for a license to manufacture a vaccine by submitting a product license application pla.
We need to continually adapt production process to satisfy evolving regulatory demand which varies country by country. In phase ii the clinical study is expanded and vaccine is given to people who have characteristics such as age and physical health similar to those for whom the new vaccine is intended. Clinical development is a three phase process.
Viruses can be lipid coatedenveloped or non enveloped. Successful manufacturing of high quality vaccines requires international standardization of starting materials production and quality control testing and the setting of high expectations for regulatory oversight of the entire manufacturing process from start to finish all while recognizing that this field is in constant change 1. Production of mature dendritic cells.
Inactivation of microorganism 22. During phase i small groups of people receive the trial vaccine. This production method requires large numbers of chicken eggs to produce vaccine and may take longer than other production methods.
The production of a vaccine can take between 6 and 36 months vaccines manufacturing is a biological process where a very high level of expertise is required.
More From Vaccine Phase 1 2 3
- Vaccine Adjuvants Mechanism Of Action
- Pfizer Covid Vaccine Dosage
- Anime App Icons Iphone Stocks
- Dow Jones Chart History One Year
- Vaccine Development Process Pdf
Incoming Search Terms:
- Http Www Who Int Influenza Vaccines Plan Resources Malone Pdf Vaccine Development Process Pdf,
- Chapter 79 Pharmaceutical Industry Vaccine Development Process Pdf,
- Supply Chain Planning For Vaccine Manufacturer With Simulation Software Anylogic Simulation Software Vaccine Development Process Pdf,
- The Purity Tests Of Vaccines Creative Biolabs Vaccine Development Process Pdf,
- So Many Candidates So Little Time Can The World Find A Good Covid 19 Vaccine Quickly Enough Briefing The Economist Vaccine Development Process Pdf,
- In The Given Diagram Of Vaccine Manufacturing Process A Is Digram Down In Photo Brainly In Vaccine Development Process Pdf,