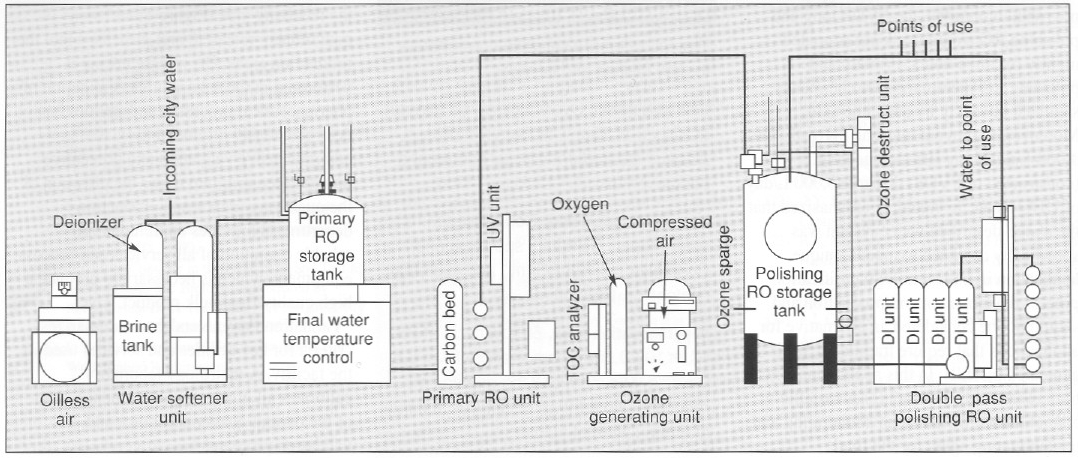
Vaccine Manufacturing Facility Layout, Figure 1 From Good Manufacturing Practices Production Of A Purification Free Oral Cholera Vaccine Expressed In Transgenic Rice Plants Semantic Scholar
Vaccine manufacturing facility layout Indeed lately is being sought by users around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view image and video data for inspiration, and according to the name of the article I will talk about about Vaccine Manufacturing Facility Layout.
- Https Absaconference Org Wp Content Uploads 2016 10 Absa2016 Session13 Schubert Pdf
- Conceptual Layout For A Vaccine Factory Download Scientific Diagram
- Facility Information Vaccine And Cell Therapy Laboratory
- Facility Design
- Bioprocess Architecture Design Of Biopharmaceutical And Vaccine Manufacturing Facilities By Michael C Flickinger
- Strategies To Overcome Modular Design Challenges
Find, Read, And Discover Vaccine Manufacturing Facility Layout, Such Us:
- Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International
- Pdf Design Of A Multi Use Dna Vaccine Production Facility
- Designing Facilities For Aseptic Filling
- Facility Design And Process Utilities Sciencedirect
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrsx1lm3krskns4br0obza4gib3amai0wjecjg0oyhqrnyeikt9 Usqp Cau
If you re searching for Vaccine Kya Hai Samjha Do you've reached the right location. We have 104 images about vaccine kya hai samjha do adding pictures, pictures, photos, wallpapers, and much more. In such webpage, we also provide number of images available. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.
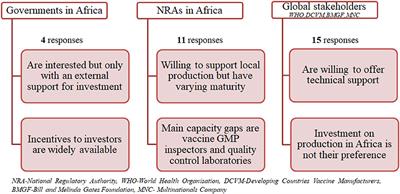
Design for an economical aids vaccine production facility c ompo sed of production quality se rvice and administrative modules along a ce ntral spine with linear product process and personnel.

Vaccine kya hai samjha do. A wellconceived design ensures a smooth regulatory review and provides for consistent reliable and contaminationfree operations. The discovery that dcs can be generated from monocytes or cd34 precursors permitted harvesting of these rare cells in large numbers for. Some of the notable vaccines made by bavarian nordic are imvamune mva bn filo mva bn hpv and mba bn rsv.
Antonia in gene therapy of cancer third edition 2014. The base case facility layout for bulk vaccine manufacturing includes defined modules for material staging and dispensing media and buffer preparation and storage upstream processing downstream processing as well as sufficient space for all support functions and appropriate airlocks and corridors permitting unidirectional flow of operators. Vaccine manufacturing innovation centre vmic in oxfordshire will become the first ever facility dedicated for the development and manufacturing of vaccines in the uk upon its completion.
The design of a vaccine manufacturing facility is critical in ensuring timely launch and reliable supply. Facilities required are such that their cost could not be reasonably borne by production of rabies vaccine alone. 1 shows the range and relative production complexity of various vaccines and vaccine types at one end is live attenuated oral polio vaccine with significantly lower cost of goods sold cogs while at the other end is the highly complex pneumococcal conjugate vaccine.
Production of mature dendritic cells. The first step in ex vivo vaccine production is the generation of an enriched population of mature dendritic cells for vaccines. This new production facility expands takedas global footprint in vaccine manufacturing beyond hikari japan and reinforces our capability to manufacture at scale and meet the global demand that we anticipate for this vaccine said rajeev venkayya md president of the global vaccine business unit at takeda.
6282016 65544 am. The design of vaccine manufacturing facilities requires understanding of regulatory business and. Global influenza vaccine market 2018 2022.
Cgmp facility design for vaccines author. Bavarian nordic also has a rd facility at martinsried germany and employs around 500 professionals across the globe. Vmic will be used to develop vaccines against serious infectious diseases such as influenza ebola zika and lassa fever.
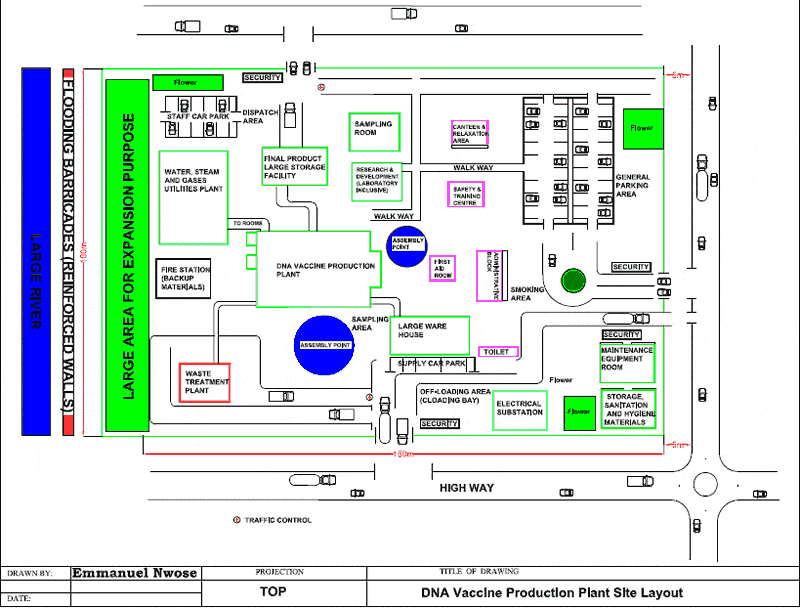
The production site it should of course be borne in mind that before any construction work begins the site must be properly surveyed even for a simple extension to existing buildings.
More From Vaccine Kya Hai Samjha Do
- Covid19 Vaccine Update
- Vaccine Schedule For Babies
- Pfizer Stock Forecast Zacks
- Coronavirus Vaccine News In Hindi Oxford University
- Pfizer Vaccine Announcement Date
Incoming Search Terms:
- Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International Pfizer Vaccine Announcement Date,
- Facility Design And Process Utilities Sciencedirect Pfizer Vaccine Announcement Date,
- Plant Design And Manufacturing Principles In Dna Vaccine Production Pfizer Vaccine Announcement Date,
- Flexible High Containment Vaccine Manufacturing Pfizer Vaccine Announcement Date,
- Vaccine Manufacturing Plant Design Constructio Pfizer Vaccine Announcement Date,
- Process Architects Bringing Value To Pharmaceutical Projects Pharmaceutical Engineering Pfizer Vaccine Announcement Date,