Vaccine Manufacturing Facility Design, Cell Based Vaccine Production Process Single Use Factory Concept Youtube
Vaccine manufacturing facility design Indeed lately has been sought by users around us, maybe one of you personally. Individuals are now accustomed to using the net in gadgets to view image and video information for inspiration, and according to the name of the article I will talk about about Vaccine Manufacturing Facility Design.
- Bioreactor Designs Respond To Evolving Needs In Vaccine Manufacturing Lab Manager
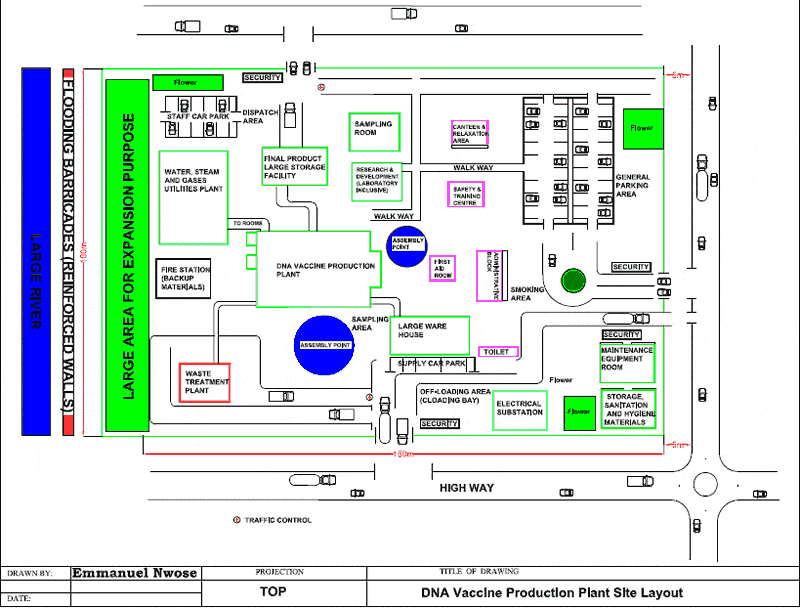
- Plant Design And Manufacturing Principles In Dna Vaccine Production
- Bio Pharmaceutical Plant Design
- Uk Government Invests In Livingston Facility To Bolster Vaccine Manufacturing Capacity Gov Uk
- Strategies To Overcome Modular Design Challenges
- Coronavirus Vaccines The Billion Dose Dash
Find, Read, And Discover Vaccine Manufacturing Facility Design, Such Us:
- Qpath Biopharmacutical Multiproduct Facility A Vaccine Example
- Vaccine Manufacturing Plant Design Constructio
- Developing Covid 19 Vaccines At Pandemic Speed Nejm
- Pdf Vaccine Scale Up And Manufacturing
- Emerging Technologies For Low Cost Rapid Vaccine Manufacture Kis 2019 Biotechnology Journal Wiley Online Library
If you re looking for Varicella Vaccine On Immunization Record you've arrived at the right place. We ve got 104 graphics about varicella vaccine on immunization record adding images, pictures, photos, backgrounds, and much more. In these web page, we additionally provide number of images available. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.
You want the contractor at the table as early as possible said molen who has been in construction for nearly 30 years and has focused.

Varicella vaccine on immunization record. Some of the notable vaccines made by bavarian nordic are imvamune mva bn filo mva bn hpv and mba bn rsv. The design of vaccine manufacturing facilities requires understanding of regulatory business and. Viral vaccine manufacturing facilities.
The new vaccine manufacturing facility subject to planning approval will be located within the wuxi biologics campus adjacent to the factory of the future biologics drug substance. The design of a vaccine manufacturing facility is critical in ensuring timely launch and reliable supply. Other areas that need to be considered in vaccine manufacturing facility design include the facilitys humidity air pressure temperature and air ventilation.
Bavarian nordic also has a rd facility at martinsried germany and employs around 500 professionals across the globe. Single use technology sut modular design can reduce factory set up time and complexity. The vaccine manufacturing facility represents a significant fixed and ongoing maintenance cost for the vaccine manufacturer.
There is currently considerable pressure on vaccine manufacturers worldwide to reduce the overall cost including facility costs of manufacturing vaccine products to enable greater access to these important products in third world and emerging markets 3 6in addition the growing demand for in countryfor country production and the. Courtesy of merck kgaa germany. The facility needs to be safe reliable and consistent to reach this goal molen and mccarthy are not just involved in construction but are part of the vaccine manufacturing facility s design.
3 a new paradigm for vaccine manufacturing facilities. Global influenza vaccine market 2018 2022. Cgmp facility design for vaccines author.
Only by evaluating the various risks involved in the project can rational and optimal choices be made regarding facility design and construction. A wellconceived design ensures a smooth regulatory review and provides for consistent reliable and contaminationfree operations. Traditionally manufacturers focus on identifying installed capacity for a particular production process to serve a specific market need.
Designing a large scale gmp production facility for biological production requires various types of risk. View related market report sample. Despite the temperature concerns facilities are not located in any particular part of the country.
Modern facility design can reduce factory set up costs and time. The wrong temperature of a facility could ruin a batch of medicine.

Building A World Class Vaccine Manufacturing Plant In Bangladesh Challenges And Opportunities Pdf Free Download Varicella Vaccine On Immunization Record
More From Varicella Vaccine On Immunization Record
- Hepatitis B Vaccine Administration Route
- Down Arrow Clipart Black And White
- Zoster Vaccine Schedule
- Vaccine Jobs
- Pfizer Covid Vaccine Wsj
Incoming Search Terms:
- Vaccine Manufacturing Plant Design Constructio Pfizer Covid Vaccine Wsj,
- Frontiers Vaccine Production In Africa A Feasible Business Model For Capacity Building And Sustainable New Vaccine Introduction Public Health Pfizer Covid Vaccine Wsj,
- A Project To Develop And Protect Features The Chemical Engineer Pfizer Covid Vaccine Wsj,
- Https Www Dcvmn Net Img Pdf Concept Design For Vaccine Facilities Dcvmn 001 Handouts Pptx Pdf Pfizer Covid Vaccine Wsj,
- Wuxi Vaccines To Build A 240m Manufacturing Facility In Ireland Pfizer Covid Vaccine Wsj,
- Closed Process An Overview Sciencedirect Topics Pfizer Covid Vaccine Wsj,