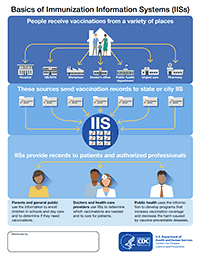
Vaccine Management Plan 2020, Vaccine Storage And Cold Chain Management Immunisation Programs
Vaccine management plan 2020 Indeed recently has been hunted by consumers around us, maybe one of you. Individuals are now accustomed to using the net in gadgets to see video and image information for inspiration, and according to the name of this post I will talk about about Vaccine Management Plan 2020.
- Ascia Action Plan Anaphylaxis Australasian Society Of Clinical Immunology And Allergy Ascia
- Deutsche Post Dhl Group Sep 03 2020 Delivery Of Covid 19 Vaccine Dhl Study Shows How Public And Private Sector Can Partner For Success
- National Vaccine Storage And Handling Guidelines For Immunization Providers 2015 Canada Ca
- Http Www Healthvermont Gov Sites Default Files Documents Pdf Id Iz Infohcp S 26h Vaccinemanagementplan Pdf
- Https Www Hhs Gov Sites Default Files Strategy For Distributing Covid 19 Vaccine Pdf
- Https Eziz Org Assets Docs Imm 1240 Pdf
Find, Read, And Discover Vaccine Management Plan 2020, Such Us:
- Colorado Unveils Draft Plan For Who Will Get A Coronavirus Vaccine First The Colorado Sun
- Covid Vaccine Complex Distribution Supply Chain Will Follow Approval
- Vaccine Storage And Handling Update Ppt Video Online Download
- Https Www Dhhs Nh Gov Dphs Immunization Documents Vmanagementplan Pdf
- 2
If you are looking for Vaccine Development Time you've reached the right location. We have 104 images about vaccine development time including images, photos, pictures, wallpapers, and more. In these page, we additionally have variety of images out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.
Arizona covid 19 vaccination plan page 7 october 2020 are responsible for reviewing the cdc advisory committee on immunization practices acip and national academies of sciences engineering and medicine nasem guidance and providing recommendations to the department to develop a state vaccine allocation plan.
Vaccine development time. Office and vaccine coordinator information. Vaccine management plan 1. Doh 348 223 may 2020.
Stand alone freezers in which vaccines are stored will be capable of maintaining. Vaccine management plan. Its purpose is to provide technical advice on key topics related to immunization logistics to help countries develop and refine national policies.
In the pre licensure phase to october 2020 the working group will outline a plan for communicating the benefits and risks of vaccines and identify approaches to messaging about vaccine safety. This section highlights key duties of designated vaccine management staff. The 2020 vaccine storage and handling toolkit is a comprehensive guide that reflects best practices for vaccine storage and handling from advisory committee on immunization practices acip recommendations product information from vaccine manufacturers and scientific studies.
Vaccine management plan page. Skip directly to site content skip directly to page options skip directly to a z link skip directly to a z link skip directly to a z link. A complete and up to date vaccine management plan providers agree to complete and maintain a vaccine management plan vmp word version that covers routine and emergency situations.
2020 vaccines for children vfc vaccine management plan standard operating procedures sops template indiana state department of health immunization division. To request this document in another format call 1 800 525 0127. The vaccine management handbook a component of the evm initiative has been written for decision makers at national and subnational levels.
In the pre launch phase gacvs will assess technical information on potential safety concerns for each vaccine and provide guidance on communication. Cdc has developed a number of publications and a toolkit that describe proper vaccine storage and handling procedures. Stand alone refrigerators where vaccines are stored will be capable of maintaining temperatures between 360 f to 460 f 20 c to 80 c.
This vaccine management plan will be updated at least annually or with any change in personnel. Vaccine management personnel. C v p.
Deaf or hard of hearing customers please call 711 washington relay or email civilrightsatdohwagov. 2020 vaccine management plan. This plan must be reviewed and updated annually every 12 months or when key staff change primaryback up.
More From Vaccine Development Time
- Pfizer Vaccine Trials Covid
- Covid Vaccine List
- Pneumococcal Vaccine Uk Green Book
- Vaccine Hepatitis B Schedule
- Up Down Arrow Icon Png
Incoming Search Terms:
- Https Www Pharmacist Com Sites Default Files Audience Aphavaccinestorage0920 Pdf Up Down Arrow Icon Png,
- Preparing For The Covid 19 Vaccine And Considerations For Mass Distribution National Governors Association Up Down Arrow Icon Png,
- Https Www In Gov Isdh Files 2020 20vaccine 20management 20plan Pdf Up Down Arrow Icon Png,
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcs0i Sbbbfujvsdotwwu1uvleknvqjg0z3zxhybuzplf1o7ngrg Usqp Cau Up Down Arrow Icon Png,
- Lbyqfjqs L7zrm Up Down Arrow Icon Png,
- Covid 19 The Most Complicated Vaccine Campaign Ever The Atlantic Up Down Arrow Icon Png,