Vaccine Development Stages Uk, Developing Covid 19 Vaccines At Pandemic Speed Nejm
Vaccine development stages uk Indeed recently has been hunted by users around us, perhaps one of you. Individuals now are accustomed to using the internet in gadgets to view image and video information for inspiration, and according to the name of the post I will discuss about Vaccine Development Stages Uk.
- Oxford Vaccine Trial To Be Tested On People From Thursday Reveals Matt Hancock
- What Covid Vaccines Are Available In The Uk
- Developing Covid 19 Vaccines At Pandemic Speed Nejm
- The Covid 19 Vaccine Development Landscape
- Oxford And Astrazeneca Resume Coronavirus Vaccine Trial
- Impact Of Hpv Vaccination And Cervical Screening On Cervical Cancer Elimination A Comparative Modelling Analysis In 78 Low Income And Lower Middle Income Countries The Lancet
Find, Read, And Discover Vaccine Development Stages Uk, Such Us:
- Covid 19 Inside The Multibillion Dollar Race For A Vaccine
- Coronavirus Vaccine Tracker The New York Times
- How Long Does It Take To Develop A Vaccine World Economic Forum
- Challenge Trials The Controversial Path To A Covid Vaccine
- How We Develop New Medicines Gsk
If you re looking for Dow Jones Futures Today Chart you've reached the perfect place. We have 100 graphics about dow jones futures today chart including images, photos, photographs, backgrounds, and much more. In such web page, we also have variety of graphics out there. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, transparent, etc.
Prioritisation of vaccine development decision making guide this guide is split into three sections technical feasibility public health value and time scale and cost of development.
Dow jones futures today chart. All the information and data collected during development and trials of a new vaccine are presented to relevant regulators at regional level where appropriate for example in the eu and national level. Alongside vaccine development doctors are trialling existing drugs for viruses such as ebola malaria and hiv. Ebel in international encyclopedia of public health 2008.
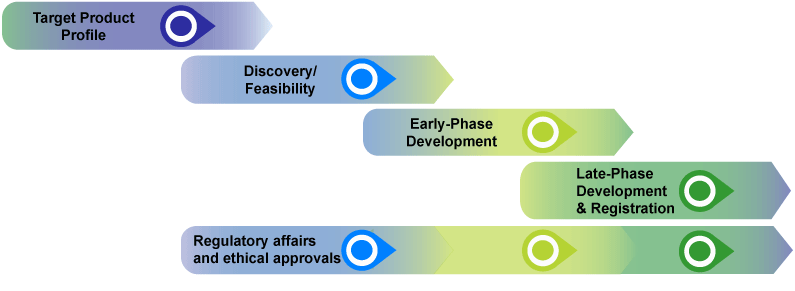
The good news is that there are dozens of plausible candidates in development with six in the late stages of the large scale clinical trials needed to prove efficacy. The standard for testing and monitoring of vaccines is higher than it is for most other medicines. These two process maps were developed by the uk vaccine rd network to show key stages in both human vaccine development and veterinary vaccine development up to phase iii clinical trials or veterinary field trialsthe network is supporting vaccine rd against some of the worlds deadliest outbreak infectious diseases and these process maps are intended to support network members in.
This is especially true for new vaccines. The general stages of the development cycle of a vaccine are. Regulatory approval submitting data and information to regulators to gain approval for vaccines.
To guide you there are criterion under each main heading which are split into how feasible or how much of a priority that criterion is. Currently a successful phase i safety clinical trial has been undertaken with a live attenuated chimeric virus based on the. This page leads to other pages that describe vaccine development and testing such as basic research clinical studies side effects and adverse reactions vaccines of the future and the vaccine product approval process.
This page aims to outline the process involved in developing and licensing a vaccine for use in the uk. Vaccine development has been on an accelerated track because of the perceived failure of anti mosquito interventions in limiting the incidence and geographic spread of wnv. Significant returns on investment and companies are reluctant to invest in developing such high risk and commercially unattractive products see chapter 5.
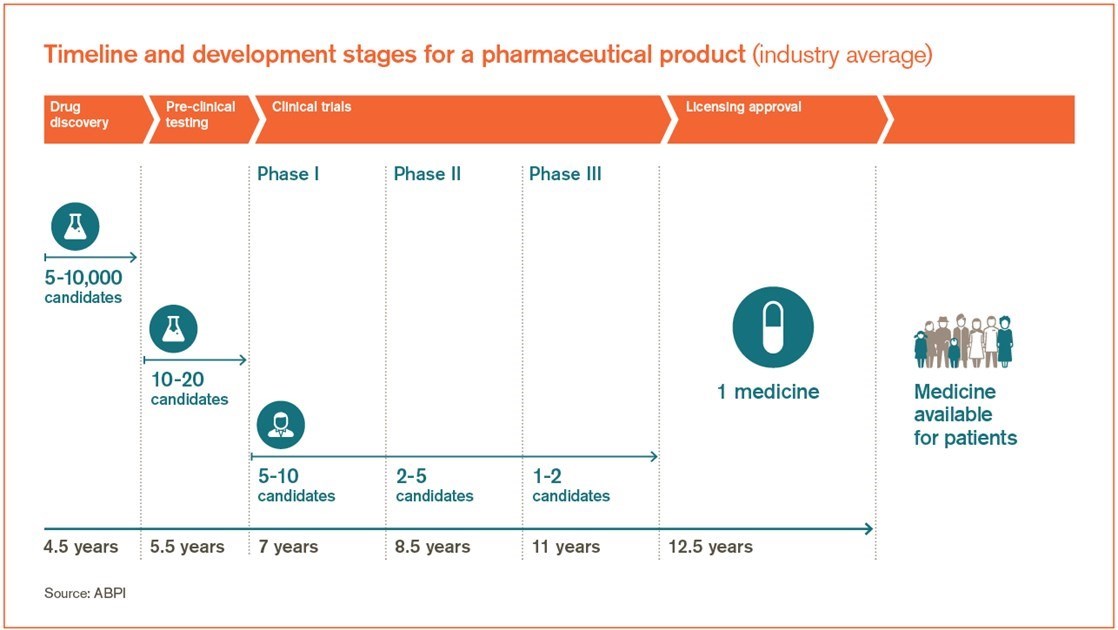
Vaccine development is a long complex process often lasting 10 15 years and involving a combination of public and private involvement. After failing to stop the spread of covid with contact tracing quarantines or lockdowns britains last hope for ending the coronavirus crisis in 2021 is a vaccine. The current system for developing testing and regulating vaccines developed during the 20 th century as the groups involved standardized their procedures and regulations.
The committee believes that priority setting and characterization of desired vaccine products is a critical stage of vaccine development particularly for vaccines of low commercial interest but acute public health need.
More From Dow Jones Futures Today Chart
- Vaccine Wont End Pandemic Reddit
- Coronavirus Vaccine Update Today In Tamil
- Vaccine Mmr
- Eva Longoria Husband Age
- Vaxigrip Influenza Vaccine Side Effects
Incoming Search Terms:
- How Long Does It Take To Develop A Vaccine World Economic Forum Vaxigrip Influenza Vaccine Side Effects,
- Respiratory Syncytial Virus Rsv Vaccine Knowledge Vaxigrip Influenza Vaccine Side Effects,
- Coronavirus Vaccine Hope Rises After A Flurry Of Positive Results New Scientist Vaxigrip Influenza Vaccine Side Effects,
- Https Www Cfr Org Backgrounder What World Doing Create Covid 19 Vaccine Vaxigrip Influenza Vaccine Side Effects,
- Accelerating Development Of Sars Cov 2 Vaccines The Role For Controlled Human Infection Models Nejm Vaxigrip Influenza Vaccine Side Effects,
- China S Military Takes Centre Stage In Covid 19 Vaccine Race Financial Times Vaxigrip Influenza Vaccine Side Effects,