Vaccine Development Process Fda, Drug Development Process Cd Biosciences
Vaccine development process fda Indeed recently has been sought by users around us, maybe one of you personally. People now are accustomed to using the internet in gadgets to see video and image data for inspiration, and according to the name of the article I will discuss about Vaccine Development Process Fda.
- Producing Prevention The Complex Development Of Vaccines Resources
- Https Disclosure Bursamalaysia Com Fileaccess Apbursaweb Download Id 106125 Name Ea Ga Attachments
- Development Approval Process Cber Fda
- Amid Speedy Trials Access To Safe Covid 19 Vaccines Is Priority Experts Vera Files
- Development Of Drugs And Vaccines Food And Drug Administration Of The Philippines
- Fda Covid 19 Vaccine Process Is Thoughtful And Deliberate Says Ex Fda Head Coronavirus Updates Npr
Find, Read, And Discover Vaccine Development Process Fda, Such Us:
- Laboratory Of Malaria Immunology And Vaccinology Vaccine Development Pipeline Nih National Institute Of Allergy And Infectious Diseases
- Fda S Critical Role In Ensuring Supply Of Influenza Vaccine Fda
- 2
- Xim6ucijflbzim
- Bill Gates Questions Fda S Credibility On Covid 19 Vaccine Cbs News
If you re searching for Apple Stock Price History Chart 2014 you've arrived at the perfect location. We have 104 graphics about apple stock price history chart 2014 including pictures, photos, pictures, backgrounds, and more. In such webpage, we also have variety of images available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, translucent, etc.
This guidance is intended to remain in effect for the duration.
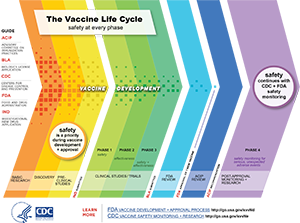
Apple stock price history chart 2014. Fda is committed to providing timely guidance to support response efforts to this pandemic. Drug development is the process of bringing a new infectious disease vaccine or therapeutic drug to the market once a lead compound has been identified through the process of drug discovery. According to guidelines established by the cdc vaccines pass through six general stages of development.
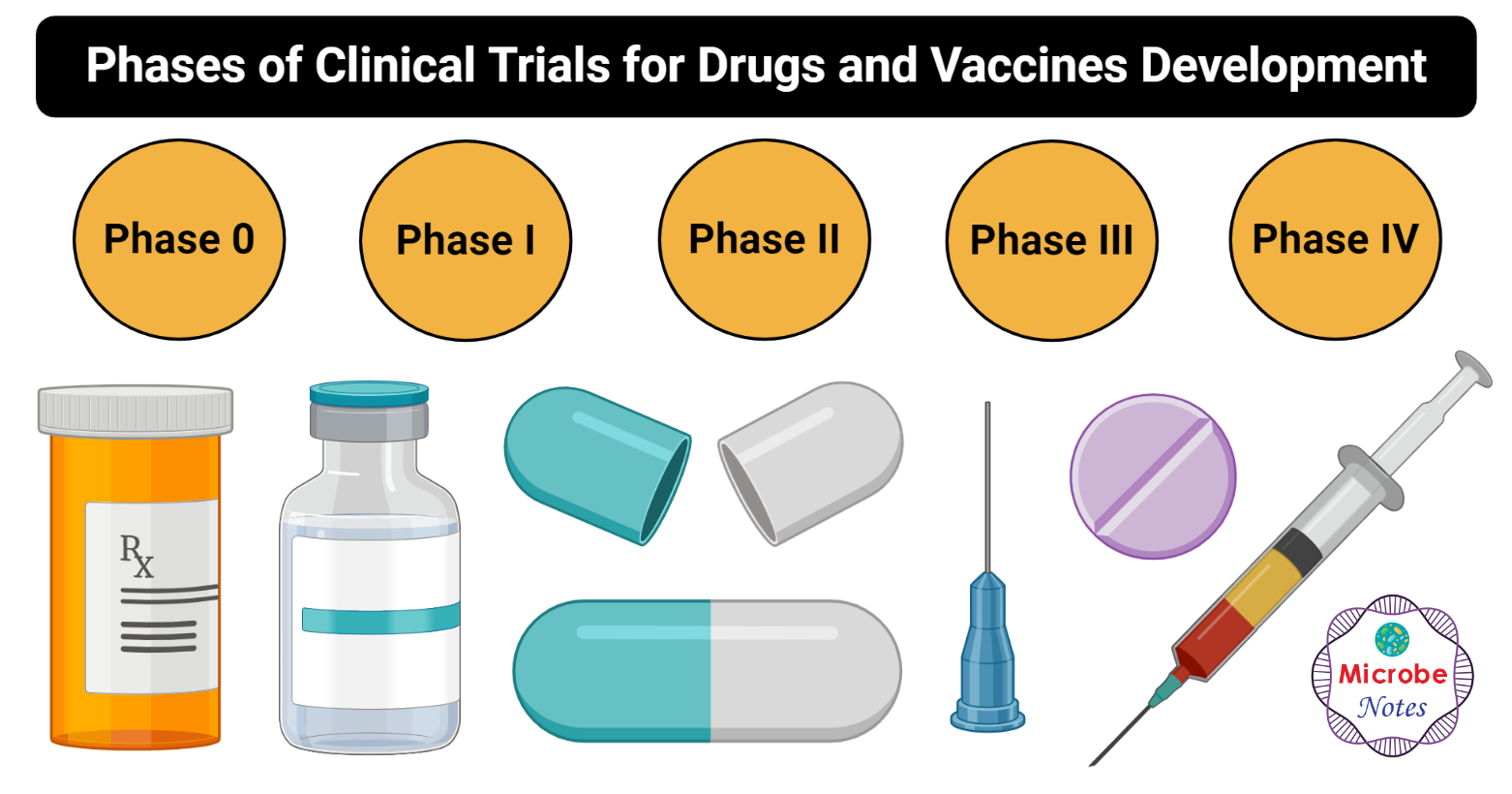
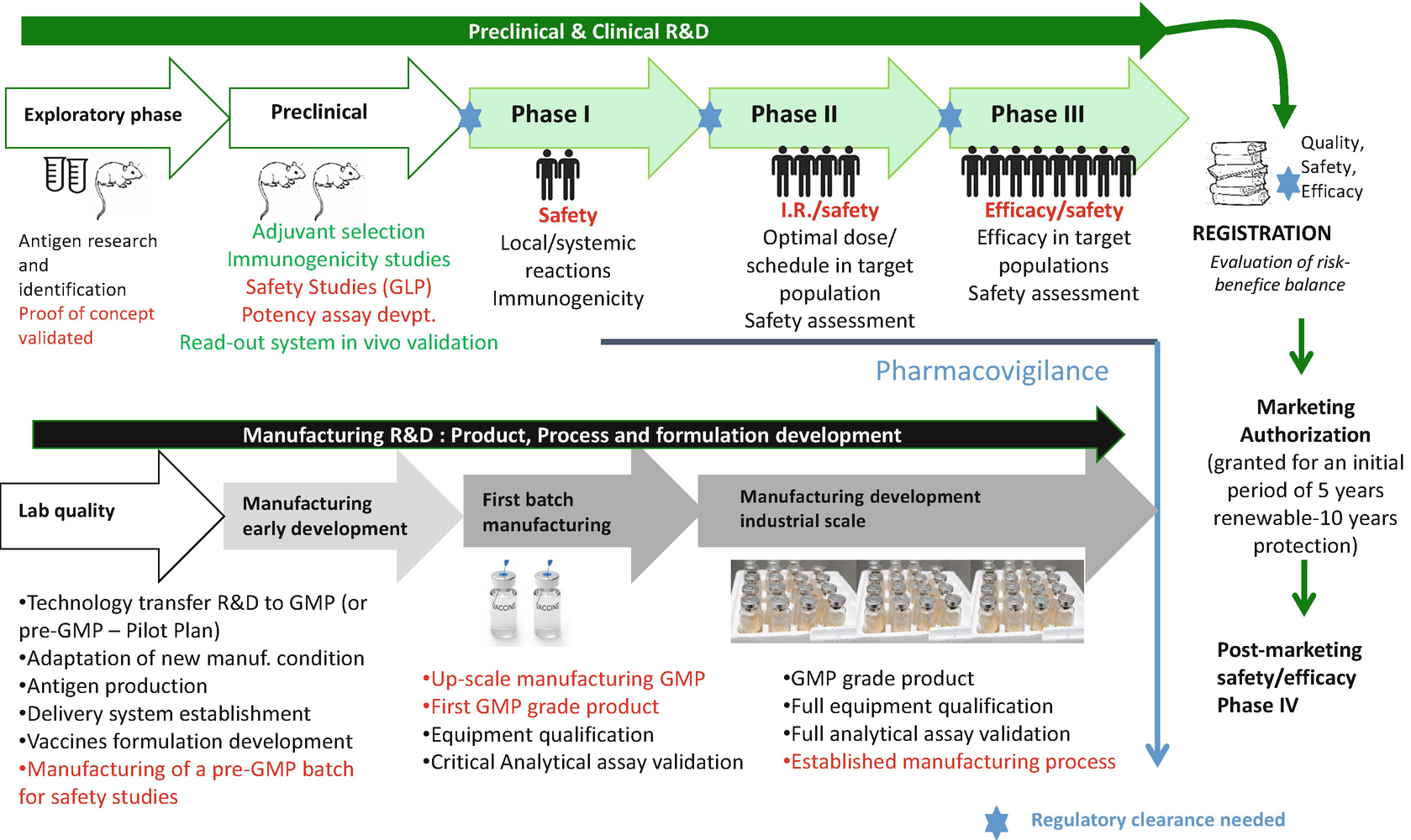
Vaccine development involves the process of taking a new antigen or immunogen identified in the research process and developing this substance into a final vaccine that can be evaluated through preclinical and clinical studies to determine the safety and efficacy of the resultant vaccine. These stages are mandated by the food and drug administration fda with its. Exploratory pre clinical clinical regulatory review and approval manufacturing and quality controlthe full process is essentially the same as the process for any drug approved for use in the united states.
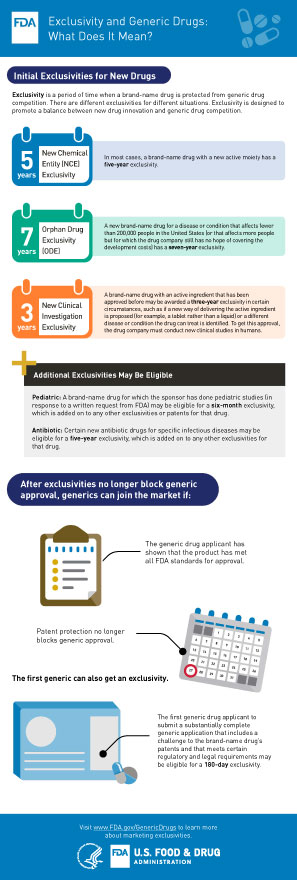
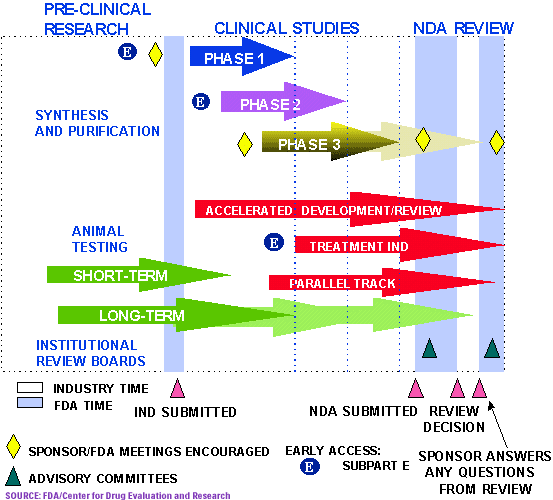
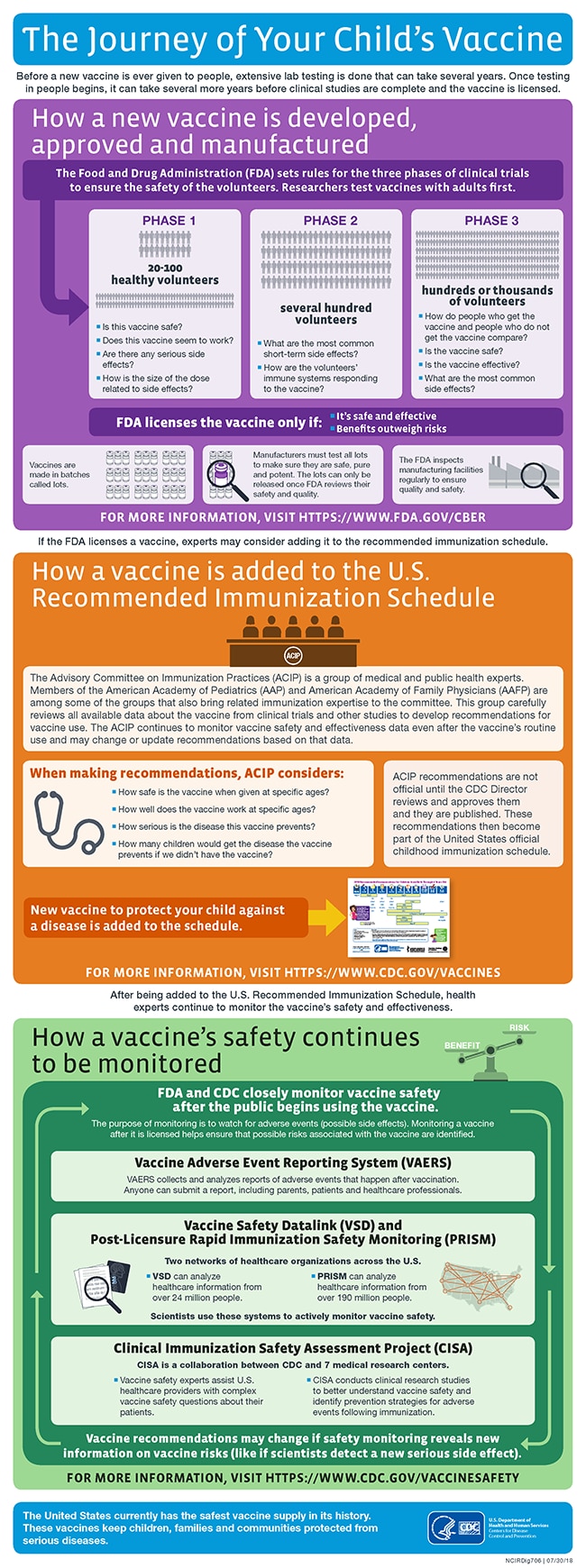
Vaccines as with all products regulated by fda undergo a rigorous review of laboratory and clinical data to ensure the safety efficacy purity and potency of these products. A sponsor who wishes to begin clinical trials with a vaccine must submit an investigational new drug. This page leads to other pages that describe vaccine development and testing such as basic research clinical studies side effects and adverse reactions vaccines of the future and the vaccine product approval process.
Vaccine clinical development follows the same general pathway as for drugs and other biologics. It includes laboratory research on microorganisms and animals filing for regulatory status such as via the fda for an investigational new drug to initiate clinical trials on humans and may include the. Development of new vaccines.
Fda is issuing this guidance to assist sponsors in the clinical development and licensure of vaccines for the prevention of covid 19. Fda experts continue to assure us that they will continue to follow a rigorous fully transparent and evidence based process to deliver a safe and effective vaccine against this virus and we must continue to do what physicians have always done review the evidence and trust the science. The general stages of the development cycle of a vaccine are.
More From Apple Stock Price History Chart 2014
- Symbol Arrow Down Signage
- Vaccine Status Icd 10
- Down Syndrome Awareness Ribbon Heart
- Vaccine Trial Phases Wiki
- Up Down Arrow Icon Png
Incoming Search Terms:
- Developing Covid 19 Vaccines At Pandemic Speed Nejm Up Down Arrow Icon Png,
- U S Vaccine Safety Overview History And How It Works Cdc Up Down Arrow Icon Png,
- Us Covid Vaccine Unlikely To Arrive Before Election Following Fda Move Coronavirus The Guardian Up Down Arrow Icon Png,
- Opinion How Long Will A Vaccine Really Take The New York Times Up Down Arrow Icon Png,
- Sars Cov 2 Vaccines In Development Nature Up Down Arrow Icon Png,
- Vaccine Testing And Approval Process Cdc Up Down Arrow Icon Png,