Vaccine Clinical Trial Timeline, First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
Vaccine clinical trial timeline Indeed lately has been hunted by users around us, maybe one of you personally. Individuals are now accustomed to using the internet in gadgets to see video and image data for inspiration, and according to the name of this article I will discuss about Vaccine Clinical Trial Timeline.
- Developing Covid 19 Vaccines At Pandemic Speed Nejm
- What S Going On In This Graph Estimated Time For Covid 19 Vaccine The New York Times
- Estimating The Cost Of Vaccine Development Against Epidemic Infectious Diseases A Cost Minimisation Study The Lancet Global Health
- Designing Tomorrow S Vaccines Nejm
- Opinion How Long Will A Vaccine Really Take The New York Times
- Vaccine Development Chart Aaf
Find, Read, And Discover Vaccine Clinical Trial Timeline, Such Us:
- Covid 19 Vaccines Treatments Timeline By Nathan Wong Towards Data Science
- Here S What Needs To Happen Before We Can All Get Vaccinated For Covid 19 Cbc News
- Https Www Cfr Org Backgrounder What World Doing Create Covid 19 Vaccine
- Covid 19 Vaccine Over To Clinical Sites To Set A Deadline For Covaxin Business Standard News
- Coronavirus Vaccine Faces Bumpy Road From Lab To Jab Voice Of America English
If you are searching for Coronavirus Vaccine Latest News India you've come to the ideal place. We ve got 100 graphics about coronavirus vaccine latest news india including pictures, photos, photographs, wallpapers, and more. In these page, we additionally have number of images out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, translucent, etc.

What S Going On In This Graph Estimated Time For Covid 19 Vaccine The New York Times Coronavirus Vaccine Latest News India

Fast Covid 19 Vaccine Timelines Are Unrealistic And Put The Integrity Of Scientists At Risk Coronavirus Vaccine Latest News India
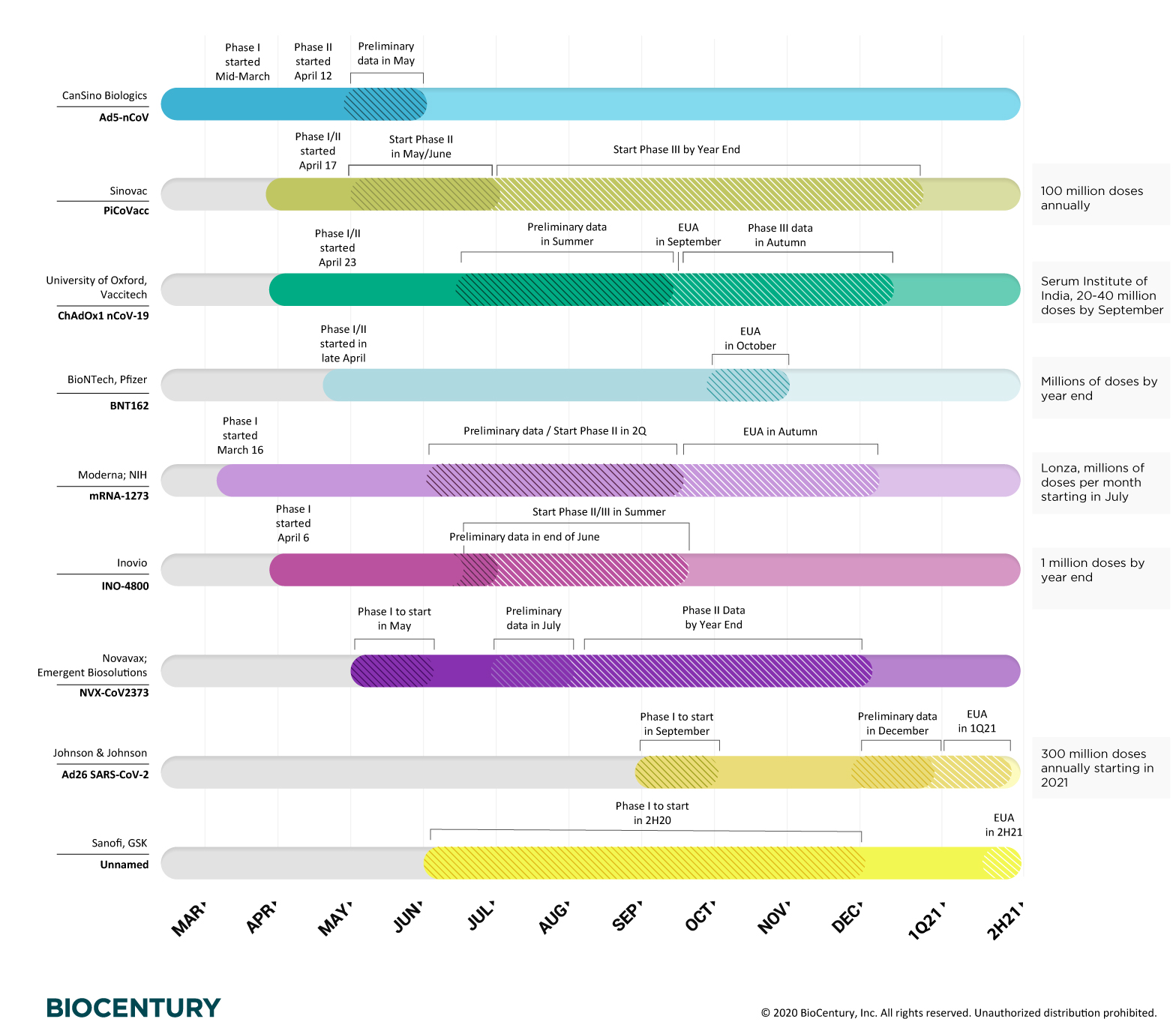
They plan to start a phase 3 trial in december and hope to know if the vaccine is safe and effective by the middle of 2021.

Coronavirus vaccine latest news india. A phase 1 clinical trial found the candidate vaccine to be safe generally well tolerated and able to induce antibodies with high levels of virus neutralizing activity. The companies launched a phase 12 clinical trial in september. During phase i small groups of people receive the trial vaccine.
Most experts believe well have several ready to distribute by. Moderna initiated phase 2 testing of the vaccine in may 2020. A vaccine trial is a clinical trial that aims at establishing the safety and efficacy of a vaccine prior to it being licensed.
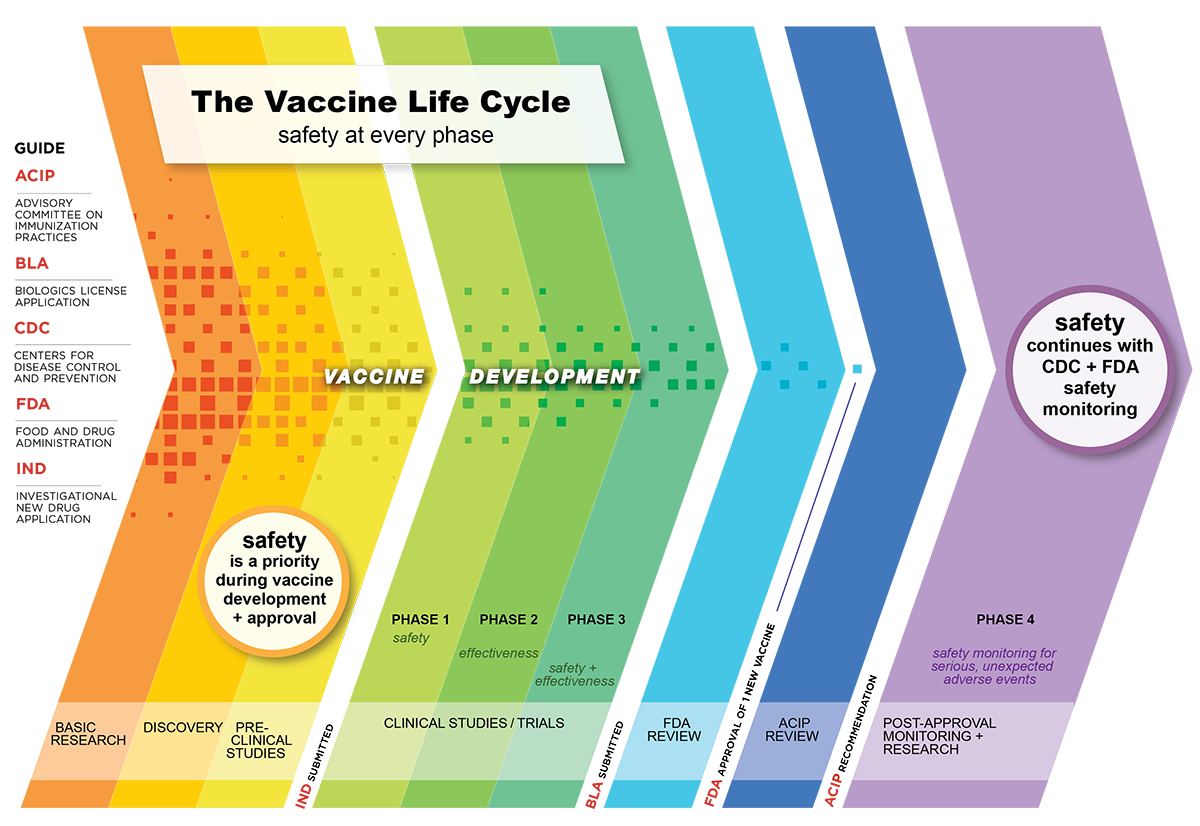
1998 first vaccine for lyme disease. 1991 first vaccine for hepatitis a. Pre marketing pre licensure vaccine clinical trials are typically done in three phases as is the case for any drug or biologic.
1981 first vaccine for hepatitis b first vaccine to target a cause of cancer 1984 first vaccine for chicken pox. A subject receives a shot in the first stage safety study clinical trial of a potential vaccine by moderna for covid 19. The investigational vaccine directs the bodys cells to express the spike protein to elicit a broad immune response.
Initial human studies referred to as phase 1 are safety and. 1998 first vaccine for rotavirus. Currently there are 59 coronavirus vaccines in various stages of clinical trials with a handful almost ready to apply for approval.
1985 first vaccine for haemophilus influenzae type b hib 1989 first vaccine for q fever. A vaccine candidate drug is first identified through preclinical evaluations that could involve high throughput screening and selecting the proper antigen to invoke an immune response. In phase iii the vaccine is given to thousands of people and tested for efficacy and safety.
In phase ii the clinical study is expanded and vaccine is given to people who have characteristics such as age and physical health similar to those for whom the new vaccine is intended. Sanofi a french biopharmaceutical company expects to begin clinical trials late this year for a covid 19 vaccine that it repurposed from work on a sars vaccine. If successful the vaccine could.
More From Coronavirus Vaccine Latest News India
- Vaccine Distribution Supply Chain
- Astrazeneca Vaccine Update Australia
- Covid19 Vaccine Watch
- Coronavirus Vaccine Latest Update In Usa
- Road Signs Arrow Up And Down
Incoming Search Terms:
- How Covid 19 Has Accelerated The Development Of Vaccines And Medicinal Therapies Lessons For The Future Of Regulation Thoughts From The Centre Deloitte Uk Road Signs Arrow Up And Down,
- The Covid 19 Vaccine Development Multiverse Nejm Road Signs Arrow Up And Down,
- On Pins And Needles Will Covid 19 Vaccines Save The World Mckinsey Road Signs Arrow Up And Down,
- Profits And Pride At Stake The Race For A Vaccine Intensifies The New York Times Road Signs Arrow Up And Down,
- Speeding Coronavirus Vaccine Development With Challenge Trials Vox Road Signs Arrow Up And Down,
- Xim6ucijflbzim Road Signs Arrow Up And Down,