Quadrivalent Influenza Vaccine Sanofi Pasteur, Jual Vaksin Influenza Fluquadri Jakarta Utara Sss Online Shop Tokopedia
Quadrivalent influenza vaccine sanofi pasteur Indeed lately has been hunted by consumers around us, maybe one of you personally. People now are accustomed to using the net in gadgets to see video and image data for inspiration, and according to the title of this article I will discuss about Quadrivalent Influenza Vaccine Sanofi Pasteur.
- Sanofi Pasteur 4928141510 Mckesson Medical Surgical
- Sanofi Fuels Competition In 4 Strain Flu Vaccine Market Pharma 기사본문 Kbr
- New Influenza Vaccine Facility First Of Its Kind In France
- Some Sanofi Flu Vaccination Deliveries Delayed By Up To Two Weeks Chemist Druggist
- Sanofi Pasteur 49281041850 Mckesson Medical Surgical
- Inkl 75 000 Doses Of Sanofi Pasteur Flu Vaccine From Batch Containing Impurities Have Been Used In Hong Kong South China Morning Post
Find, Read, And Discover Quadrivalent Influenza Vaccine Sanofi Pasteur, Such Us:
- Http Www Cpwy Org Doc 2034 Pdf
- These Highlights Do Not Include All The Information Needed To Use Fluzone Quadrivalent Safely And Effectively See Full Prescribing Information For Fluzone Quadrivalent Fluzone Quadrivalent Influenza Vaccine Suspension For Intramuscular Injection
- Sanofi Ships First Flu Vaccines For 2018 2019 Season
- Sanofi Pasteur 49281041850 Mckesson Medical Surgical
- Pdf Impact Of Quadrivalent Influenza Vaccines In Brazil A Cost Effectiveness Analysis Using An Influenza Transmission Model
If you are searching for Vaccine For Coronavirus Latest Update Usa you've come to the perfect place. We ve got 104 images about vaccine for coronavirus latest update usa including pictures, photos, pictures, backgrounds, and much more. In such webpage, we additionally have number of images available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, transparent, etc.
Indications and usage fluquadri quadrivalent influenza vaccine is an inactivated quadrivalent influenza vaccine.
:no_upscale()/cdn.vox-cdn.com/uploads/chorus_image/image/67453259/merlin_2830808.0.jpg)
Vaccine for coronavirus latest update usa. Fluzone quadrivalent is a vaccine indicated for active immunization for the prevention of influenza disease caused by influenza a subtype viruses and type b viruses contained in the vaccine. Fluzone high dose quadrivalent is approved for use in persons 65 years of age. Fluzonequadrivalent is indicated for active immunization against influenza caused by the specific strains of influenza virus contained in the vaccine in adults and children 6 months of age.
Fluzone quadrivalent is approved for use in persons 6 months of age and older. Fluzone quadrivalent flublok quadrivalent and fluzone high dose quadrivalent are vaccines indicated for active immunization for the prevention of influenza disease caused by influenza a subtype viruses and influenza type b viruses contained in the vaccine. Quadrivalent influenza vaccine split virion inactivated provides active immunisation against four influenza virus strains two a subtypes and two b types contained in the vaccine.
Quadrivalent influenza vaccine split virion inactivated induces humoral antibodies against the haemagglutinins within 2 to 3 weeks. Flublok quadrivalent is approved for use in persons 18 years of age and older. For active immunization for the prevention of influenza disease caused by influenza virus subtypes a and type b.
Flublok quadrivalent is approved for use in persons 18 years of age and older. Quadrivalent influenza vaccine split virion inactivated suspension for injection in pre filled syringe patient information leaflet pil by sanofi pasteur. Quadrivalent influenza vaccine qive standard egg grown quadrivalent influenza vaccine split virion inactivated from 6 months equal to or less than 005 micrograms per 05 ml dose 0113 238 7552 sanof i pasteur vaccines quadr valent influenza vaccine qive standard egg grown quadrivalent influenza vaccine split virion inactivated from 6.
Page 4 of 34. Sanofi pasteur 02 jan 2018 v02 450 fluquadri le7199 confidential proprietary information page 1 of 11 fluquadri quadrivalent influenza vaccine types a and b subvirion 2018 formula full prescribing information. Quadrivalent influenza vaccine split virion inactivated company.
Fluzone quadrivalent is indicated for active immunization in persons 6 months of age and older. Product subject to medical prescription which may not be renewed a patient information leaflet patient information leaflet last updated on medicinesie. Influenza vaccine split virion inactivated legal category.
More From Vaccine For Coronavirus Latest Update Usa
- Biontech Stock Price Today Per Share
- Biontech Stock Forecast Walletinvestor
- Vaccination Program For Cattle In South Africa
- Homemade Covid Vaccine Questions
- Hepatitis B Vaccine Clip Art
Incoming Search Terms:
- Vaxigriptetra Full Prescribing Information Dosage Side Effects Mims Malaysia Hepatitis B Vaccine Clip Art,
- Coronavirus Vaccine Plans From John Hopkins The National Academies And More Deseret News Hepatitis B Vaccine Clip Art,
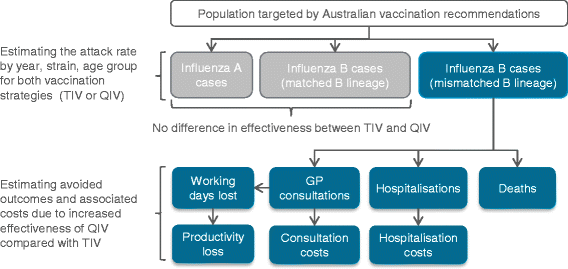
- Impact Of Quadrivalent Influenza Vaccine On Public Health And Influenza Related Costs In Australia Bmc Public Health Full Text Hepatitis B Vaccine Clip Art,
- Sanofi Pasteur Begins Shipping Its First Flu Vaccines For The Us 2018 19 Season Pharmaceutical Commerce Hepatitis B Vaccine Clip Art,
- Sanofi Pasteur 49281041550 Mckesson Medical Surgical Hepatitis B Vaccine Clip Art,
- Flublok Quadrivalent Influenza Vaccine Sanofi Flu Hepatitis B Vaccine Clip Art,