Quadrivalent Flu Vaccine Sanofi Emc, Vaksinjakarta Instagram Posts Photos And Videos Picuki Com
Quadrivalent flu vaccine sanofi emc Indeed recently is being hunted by users around us, perhaps one of you personally. People are now accustomed to using the internet in gadgets to view video and image data for inspiration, and according to the name of this post I will talk about about Quadrivalent Flu Vaccine Sanofi Emc.
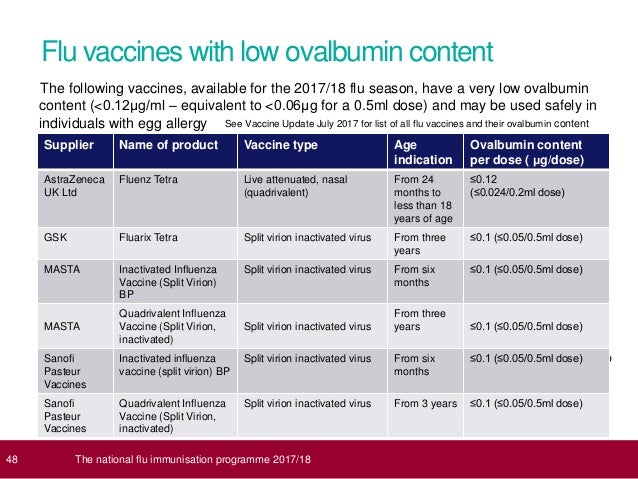
- The National Flu Immunisation Programme 2017 18 Training For Profes
- Gsk Shipping Record Number Of Flu Vaccine Doses For Upcoming Season Drug Store News
- Flu Shot During Covid 19 What To Know About The 2020 2021 Season
- Https Www England Nhs Uk South Wp Content Uploads Sites 6 2018 08 Phe 2018 19 Inactivated Influenza Vaccine Pdf
- Implementation Of The United Kingdom S Childhood Influenza National Vaccination Programme A Review Of Clinical Impact And Lessons Learned Over Six Influenza Seasons Sciencedirect
- Https Www Publichealth Hscni Net Sites Default Files 2018 08 Flu 20presentation 20with 20speaker 20notes 20pdf 20aq 20updated 20030818 Pdf
Find, Read, And Discover Quadrivalent Flu Vaccine Sanofi Emc, Such Us:
- Health Experts Want You To Get Your Flu Shot Now Here S Why
- Dell Emc Expands High Performance Computing Portfolio With Advances In Cloud Software And Systems
- Avian Flu Diary Taiwan Fda Hk Ha Reject 2 Batches Of Sanofi Flu Vaccine For Impurities
- Https Www Tandfonline Com Doi Pdf 10 1080 14760584 2017 1324302
- Frontiers Viral Emerging Diseases Challenges In Developing Vaccination Strategies Immunology
If you are looking for Vaccine Timeline Uk you've come to the right location. We have 104 images about vaccine timeline uk including images, photos, pictures, backgrounds, and more. In these web page, we also provide variety of graphics out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, transparent, etc.
Medical information e mail email protected.

Vaccine timeline uk. The spc have been updated to reflect the strain composition for the 2019 2020 season flu vaccine. You can help by reporting any side effects you may get. Quadrivalent influenza vaccine split virion inactivated suspension for injection in pre filled syringe.
For each version we show the dates it was published on emc and the reasons for change. Go advanced search sanofi pasteur. 410 thames valley park drive reading berkshire rg6 1pt uk.
Quadrivalent influenza vaccine split virion inactivated suspension for injection in pre filled syringe patient information leaflet pil by sanofi pasteur. 353 01 403 5600. Quadrivalent influenza vaccine split virion inactivated suspension for injection in pre filled syringe influenza vaccine split virion inactivated sanofi pasteur.
Last updated on emc 04 12 2019 view medicine sanofi pasteur contact details when a pharmaceutical company changes an smpc or pil a new version is published on emc. Enter medicine name. Sanofi pasteur 02 jan 2018 v02 450 fluquadri le7199 confidential proprietary information page 1 of 11 fluquadri quadrivalent influenza vaccine types a and b subvirion 2018 formula full prescribing information.
This will allow quick identification of new safety information. Quadrivalent influenza vaccine split virion inactivated patient info. Enter medicine name.
Fluzone quadrivalent is approved for use in persons 6 months of age and older. Falsified and contaminated defibrotide identified in who regions of western pacific europe and eastern mediterraneanupdate of 1 july 2020 to medical product alert n052020 this alert relates to falsified defibrotide 200mg vials of 25ml 80mgml concentrate for solution for infusion identified to date in argentina australia latvia malaysia and saudi arabia. Quadrivalent influenza vaccine split virion inactivated suspension for injection in pre filled syringe quadrivalent influenza vaccine split virion inactivated this medicine is subject to additional monitoring.
The safety of quadrivalent influenza vaccine split virion inactivated was assessed in six clinical trials in which 3040 adults from 18 to 60 years of age 1392 elderly over 60 years of age and 429 children from 9 to 17 years of age received one dose of quadrivalent influenza vaccine split virion inactivated and 884 children from 3 to 8. Indications and usage fluquadri quadrivalent influenza vaccine is an inactivated quadrivalent influenza vaccine. Trivalent influenza vaccine split virion inactivated high dose.
More From Vaccine Timeline Uk
- Planos Modelos De Casas De Dos Pisos Modernas
- Vaccine News Today Russia
- Corona Virus Vaccine In India
- Vaccine For Coronavirus Latest News
- Vaccine Years
Incoming Search Terms:
- Some Flu Shot Shipments Will Be Delayed Medical Economics Vaccine Years,
- The National Flu Immunisation Programme 2017 18 Training For Profes Vaccine Years,
- Https Www England Nhs Uk South Wp Content Uploads Sites 6 2018 08 Phe 2018 19 Inactivated Influenza Vaccine Pdf Vaccine Years,
- Https Www Communitypharmacy Scot Nhs Uk Media 2150 Pgd Nos Influenza Pdf Vaccine Years,
- The Best Time To Get Your Flu Shot Hint It S Not Right Now Wthr Com Vaccine Years,
- Fluzone Quadrivalent Influenza Vaccine Sanofi Flu Vaccine Years,