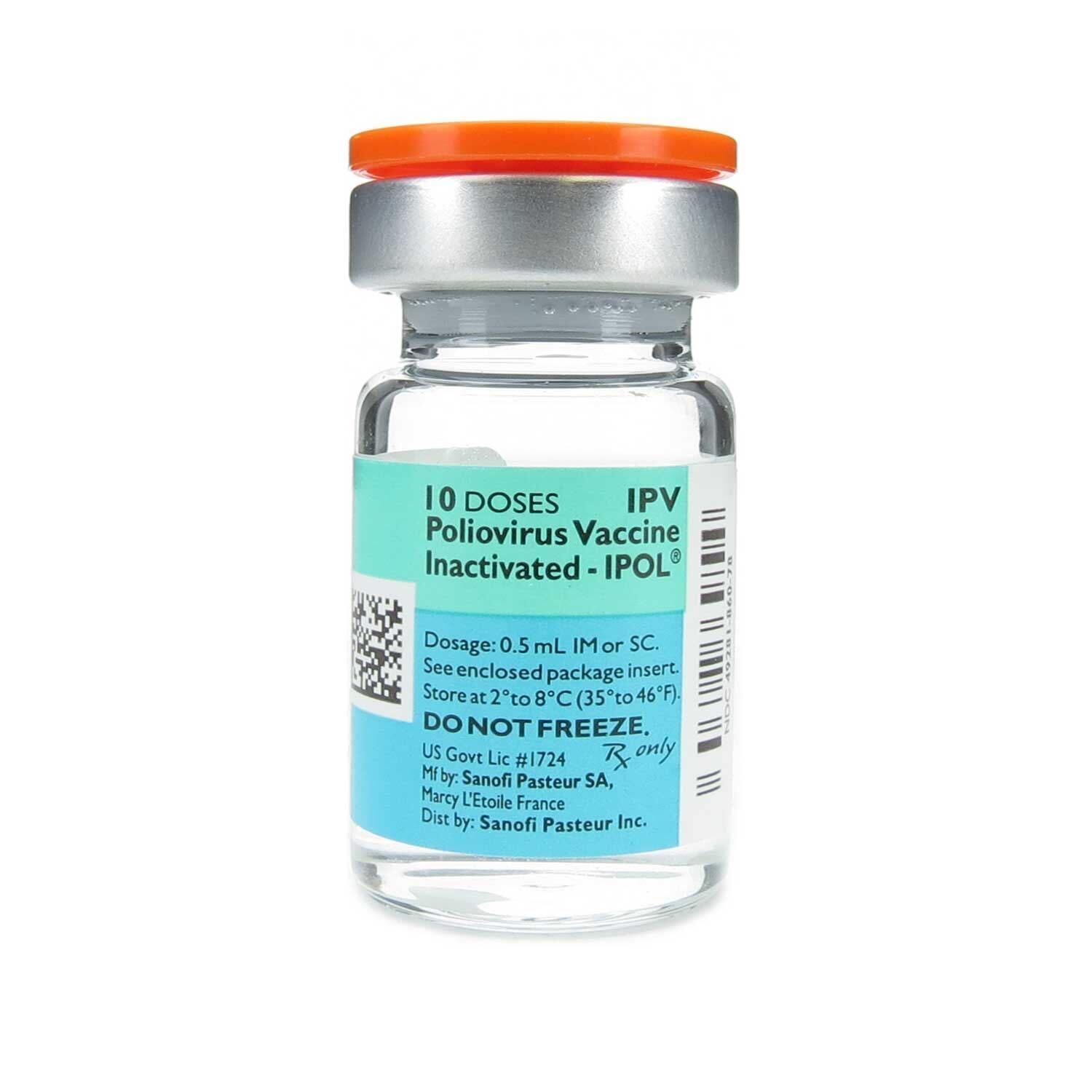
Polio Vaccine Ipv Package Insert, Https Www Fda Gov Files Vaccines 2c 20blood 20 26 20biologics Published Package Insert Prevnar 13 Pdf
Polio vaccine ipv package insert Indeed lately has been sought by consumers around us, perhaps one of you. People now are accustomed to using the internet in gadgets to see image and video data for inspiration, and according to the title of this article I will discuss about Polio Vaccine Ipv Package Insert.
- Read The Fine Print Vaccine Package Inserts Reveal Hundreds Of Medical Conditions Linked To Vaccines Children S Health Defense
- Sample Revised Vis For Polio With Informed Consent Section Added
- Time To Take The Shot Before The Back To School Buzzer News Ocala Com Ocala Fl
- Https Idph Iowa Gov Portals 1 Userfiles 39 Quadracel 20vaccine 20summary 20final Pdf
- Dtap Ipv Kinrix Vaccine Ingredients
- 2
Find, Read, And Discover Polio Vaccine Ipv Package Insert, Such Us:
- Oral Polio Vaccine Scarsdale Medical Group
- Ipol Poliovirus Vaccine 5ml Mdv Clint Pharmaceuticals
- Https Www Vaccineshoppe Com Image Cfm Doc Id 13799 Image Type Product Pdf
- Dtap Ipv Kinrix Vaccine Ingredients
- Vaccine Guide Cdc Vaccine Excipient And Media Summary
If you are looking for Post Exposure Rabies Vaccine you've arrived at the ideal place. We have 104 images about post exposure rabies vaccine including pictures, photos, photographs, wallpapers, and more. In these page, we also provide variety of graphics out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.
Ipol vaccine and dtp was administered to more than 3000 infants between 2 to 18 months of age using ipv only schedules and immunogenicity data are available from 1485 infants.
Post exposure rabies vaccine. The recommended dose for both children and adults is 05 ml. Single antigen ipv ipol is distributed in single dose syringes or in 10 dose vials. However only ipol is still used in the united states.
After two doses of vaccine given during the rst year of life seroprevalence rates for detectable serum neutralizing. Anthrax dtaptdaptd hepatitis a hepatitis b hib hpv influenza meningococcal mmrmmrv pneumococcal polio rotavirus smallpox varicellaherpes zoster yellow fever. Sanofi pasteur country of manufacture.
Polio vaccine injectable manufacturer. Ipol vaccine is indicated for active immunization of infants as young as 6 weeks of age children and adults for the prevention of poliomyelitis caused by poliovirus types 1 2 and 3. Two inactivated polio vaccine ipv products are licensed in the united states.
Imovax polio vaccine type. France date of prequalification. Intramuscular or subcutaneous vaccine vial monitor.
Poliomyelitis vaccine in line with the national vaccination program. 36 months at 2 8 0c. Type 1 mahoney type 2 mef 1 and type 3 saukett.
Poliovax has been discontinued. Poliovirus vaccine inactivated produced by sanofi pasteur sa is a sterile suspension of 4 three types of poliovirus. Type 7 shelf life.
1 month before departure particularly when their last immunization was more than 15 years ago. Persons fully immunized against poliomyelitis and leaving to areas with a high incidence of poliomyelitis are advised to re vaccinate with a single dose of polio vaccine approx. Package insert pdf 162mb.
Who package insert june 2010 1. 09 december 2005 nra of record. 10 dose vial route of administration.
Poliomyelitisvaccine multidose suspension for injection 25 ml. Qualitative and quantitative composition a 05 ml dose of vaccine contains. Polio sabin one and three oral can be administered at the same time as haemophilus influenzae type b vaccine hepatitis b vaccine diphtheria pertussis andor tetanus vaccine measles rubella andor mumps vaccine yellow fever vaccine or bcg vaccine if this fits into the vaccination schedule.
Inactivated polio virus type 1 mahoney1 40 d antigen units inactivated polio virus type 2 mef 11 8 d antigen units.
More From Post Exposure Rabies Vaccine
- Coronavirus Vaccine News Today Marathi
- Vaccine Coronavirus Update Usa
- Vaccine Japanese Encephalitis
- Covid 19 Vaccine Update News In India In Hindi
- Pfizer Covid Vaccine Phase 3 Trial
Incoming Search Terms:
- India S Immunization Program How Is Ipv Different From Opv Civilsdaily Pfizer Covid Vaccine Phase 3 Trial,
- Https Www Fda Gov Files Vaccines 2c 20blood 20 26 20biologics Published Package Insert Prevnar 13 Pdf Pfizer Covid Vaccine Phase 3 Trial,
- Flulaval Vaccine Guide National Vaccine Support Group Pfizer Covid Vaccine Phase 3 Trial,
- Fluzone Vaccine Guide National Vaccine Support Group Pfizer Covid Vaccine Phase 3 Trial,
- Dtap Ipv Kinrix Vaccine Ingredients Pfizer Covid Vaccine Phase 3 Trial,
- Dtap Ipv Hib Pentacel Vaccine Ingredients Pfizer Covid Vaccine Phase 3 Trial,