Pneumococcal Vaccine Australian Immunisation Handbook, Pneumococcal Vaccination For High Risk Adults Ppt Download
Pneumococcal vaccine australian immunisation handbook Indeed lately is being sought by consumers around us, maybe one of you personally. Individuals now are accustomed to using the net in gadgets to view video and image data for inspiration, and according to the name of this article I will discuss about Pneumococcal Vaccine Australian Immunisation Handbook.
- Pneumococcal Vaccination For Children
- Https Sydneynorthhealthnetwork Org Au Wp Content Uploads 2020 08 Immunisation Update Presentation 2020 Pdf
- 2
- Https Www Cesphn Org Au 9001 Preview Population Health Immunisation 1 3341 July 2020 Immunisation Enews File
- Https Www Immunisationcoalition Org Au Wp Content Uploads 2020 07 Ic Pneumococcal Slides 2020 An Final Pdf
- Racgp Webinars Immunisation Programs
Find, Read, And Discover Pneumococcal Vaccine Australian Immunisation Handbook, Such Us:
- Https Www Sahealth Sa Gov Au Wps Wcm Connect Public Content Sa Health Internet Resources Sharp And To The Point Issue 63 December 2019
- Influenza Vaccine Recommendations The Melbourne Vaccine Education Centre Mvec
- Table 1 From Invasive Pneumococcal Disease In Australia 2002 Semantic Scholar
- Adult Pencs Help
- Pdf 10 Valent Pneumococcal Non Typeable H Influenzae Protein D Conjugate Vaccine Phid Cv10 Versus 13 Valent Pneumococcal Conjugate Vaccine Pcv13 As A Booster Dose To Broaden And Strengthen Protection From Otitis Media Previx Boost In Australian
If you re searching for Vaccine Novavax Enters Final Largescale Testing you've reached the ideal location. We ve got 101 graphics about vaccine novavax enters final largescale testing adding pictures, photos, pictures, backgrounds, and much more. In these page, we also have number of graphics available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.
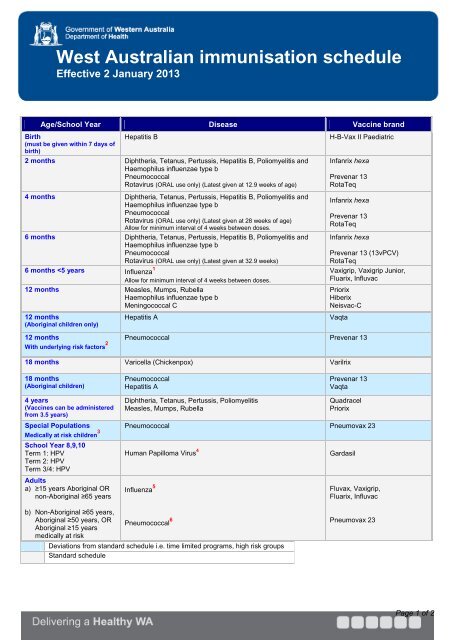
See the australian immunisation handbook for more details.

Vaccine novavax enters final largescale testing. The decision tree should be read in conjunction with the nip pneumococcal vaccination schedule from 1 july 2020. It includes information on applying key changes to the pneumococcal vaccination schedule and the list of risk conditions for pneumococcal vaccine recommendations and. Please refer to the 10th edition australian immunisation handbook1 for comprehensive listing of at risk conditions and recommendations recommended for those with risk factors for invasive disease who have never received the 13 valent conjugate vaccine.
Jackson la gurtman a van cleeff m et al. From 1 july 2020 there are changes to the national immunisation program nip pneumococcal vaccination schedule. Public consultation on the proposed changes to the recommended use of pneumococcal vaccines in the australian immunisation handbook handbook was conducted over a 4 week period from 31 january 2020 to 1 march 2020 during which time the proposed changes were available on the citizen space website.
Many children and adults with these risk conditions are eligible for funded doses of 13vpcv and 23vppv under the national immunisation program nip. The tool has been developed and designed for appropriate pneumococcal disease vaccination of adults and children in australia based on the funded national immunisation program nip scheduled vaccines and pbs funded and unfunded pneumococcal disease vaccination recommendations for individuals with category a or category b medical risk factors. The australian immunisation handbook provides clinical advice for health professionals on the safest and most effective use of vaccines in their practice.
See the australian immunisation handbook for the list of specified risk conditions that are eligible to receive free pneumococcal vaccines. Many children and adults with these risk conditions are eligible for funded doses of pneumococcal vaccines under the national immunisation program. These vaccines are funded under the national immunisation program.
Influence of initial vaccination with 13 valent pneumococcal conjugate vaccine or 23 valent pneumococcal polysaccharide vaccine on anti pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. See the australian immunisation handbook for the full list of risk conditions including which conditions are funded under the national immunisation program. Children and adults with these risk conditions are at increased risk of pneumococcal disease and would benefit from additional doses of pneumococcal vaccine.
This fact sheet provides clinical advice for vaccination providers on pneumococcal vaccination from 1 july 2020.

Pneumococcal Disease The Australian Immunisation Handbook Vaccine Novavax Enters Final Largescale Testing
More From Vaccine Novavax Enters Final Largescale Testing
- Covid Vaccine October Oxford
- Vaccine Phase Trials
- Quadrivalent Flu Vaccine Sanofi Price
- Moderna Stock Price Prediction 2020
- Vaccine Phase 3 Trials Covid
Incoming Search Terms:
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctdr9syhx64 Drhse Fqwfoxxllzjn2l21r 8j4wsnenygdfrxk Usqp Cau Vaccine Phase 3 Trials Covid,
- Table 1 From Invasive Pneumococcal Disease In Australia 2002 Semantic Scholar Vaccine Phase 3 Trials Covid,
- Pneumococcal Disease And Vaccination Recommendations The State Of Play Respiratory Medicine Today Vaccine Phase 3 Trials Covid,
- Ppt Program Support Update Powerpoint Presentation Free Download Id 6393598 Vaccine Phase 3 Trials Covid,
- Pneumococcal Disease The Australian Immunisation Handbook Vaccine Phase 3 Trials Covid,
- Https Bestongroundperformance Com Wp Content Uploads 2015 08 Australian Immunisation Guidelines For International Travellers Pdf Vaccine Phase 3 Trials Covid,