Pfizer Vaccine Trial Protocol, Xconomy Moderna Pfizer Protocols May Make Covid Vaccines Hard To Compare
Pfizer vaccine trial protocol Indeed recently is being hunted by users around us, maybe one of you personally. People are now accustomed to using the internet in gadgets to see image and video information for inspiration, and according to the name of this article I will discuss about Pfizer Vaccine Trial Protocol.
- Pfizer Modifies Protocol For Virus Vaccine Study Wbma
- The Latest Pfizer Modifies Protocol For Virus Vaccine Study Boston News Weather Sports Whdh 7news
- Pfizer Ceo All But Rules Out Covid 19 Vaccine Before Election Day The New York Times
- Ywo5zzk 9tyvdm
- Py7rbr9ywlwkbm
- Pfizer Begins Human Trials Of Possible Coronavirus Vaccine The New York Times
Find, Read, And Discover Pfizer Vaccine Trial Protocol, Such Us:
- Pfizer And Biontech Choose Lead Mrna Vaccine Candidate Against Covid 19 And Commence Pivotal Phase 2 3 Global Study Nasdaq Bntx
- What To Expect As Moderna S Covid 19 Vaccine Moves To Phase 3 Trials
- Pfizer Modifies Protocol For Virus Vaccine Study To Include 12 15 Year Olds Mysuburbanlife Com
- Coronavirus Covid 19 Vaccine India Latest Updates Icmr Bharat Covaxin Oxford Zydus Cadila Moderna Pfizer Biontech China Latest Update Usa Human Clinical Trial The Financial Express
- Pfizer And Biontech Plan To Initiate U S Covid 19 Vaccine Trial Next Week Biospace
If you are searching for Vaccine Development you've reached the right place. We have 100 images about vaccine development adding pictures, photos, photographs, backgrounds, and much more. In these web page, we additionally provide variety of graphics available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
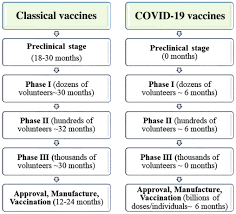
Also readpfizer modifies protocol for virus vaccine study two russian covid 19 vaccines have been registered for use even before clinical trials were completed but have not been widely accepted.
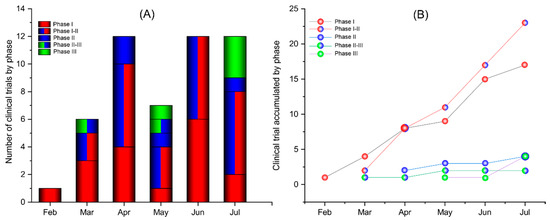
Vaccine development. 17 shortly after moderna did the same the new york times reported. Modernas vaccine candidate mrna 1273 is nearing the finish line in its push to enroll 30000 individuals in a late stage trial of a novel coronavirus vaccine. Pfizer and its partner biontech have taken the rare step of publishing the clinical protocol for their late stage covid 19 vaccine trialsthe vaccine candidate selected by the two companies for phase 23 evaluation is bnt162b2 at a dose of 30 ug administered as a 2 dose separated by 21 days schedule.
How pfizers covid 19 clinical trial is designed. Pfizers clinical trial protocol outlines for the company scientists and regulators how the drugmaker could show that its vaccine meets efficacy and safety standards set by the us. Vaccine protocols give detailed look at companies trials.
Several pharmaceutical companies have published their vaccine trial protocols. This unusually transparent action during a major drug trial deserves praise close inspection of the protocols raises. Our team of in house experts has decades of experience developing vaccines and study protocols a document that outlines the objectives design and methodology for a clinical trial to potentially help prevent serious emerging and life threatening infections and diseases.
Previously reported data from vaccination with 10 mg or 30 mg of bnt162b1 in adults 18 to 55 years of age suggested that it could be a promising covid 19 vaccine candidate. For pfizers vaccine. In september following months of campaigning for greater openness12 four manufacturers made their full study protocols publicly available3456 the publications create a rare opportunity for real time transparency.
Pfizer released the protocol for its phase 3 covid 19 vaccine trial sept. Pfizer has a deep heritage in vaccine development. The protocol details how participants are being.
More From Vaccine Development
- Vaccine For Coronavirus Update India
- Influenza Vaccine Price In Bangladesh
- Influenza Vaccine Name In Pakistan
- Biontech Ceo Cnn
- Transparent Background Corn Stalks Clipart
Incoming Search Terms:
- Pfizer Modifies Protocol For Covid 19 Vaccine Study Honolulu Star Advertiser Transparent Background Corn Stalks Clipart,
- Pfizer Follows Moderna S Suit In Releasing Covid 19 Vaccine Study Protocol Medcity News Transparent Background Corn Stalks Clipart,
- Qhp A9myhsabbm Transparent Background Corn Stalks Clipart,
- Pfizer Pfi Covid 19 Vaccine Trial Plan May Beat Rivals To Early Look Bloomberg Transparent Background Corn Stalks Clipart,
- Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html Transparent Background Corn Stalks Clipart,
- Clinical Trials Pfizer Uk Transparent Background Corn Stalks Clipart,