Pfizer Vaccine Safety Data, Pfizer Germany S Biontech Release New Data On Covid 19 Vaccine Medcity News
Pfizer vaccine safety data Indeed recently is being hunted by consumers around us, perhaps one of you. Individuals now are accustomed to using the net in gadgets to view image and video information for inspiration, and according to the name of the article I will talk about about Pfizer Vaccine Safety Data.
- Scaling Up To Manufacture And Supply A Covid 19 Vaccine If Approved Pfizer
- Pfizer Presents Phase 2 And 3 Data From Its 20 Valent Pneumococcal Conjugate Vaccine 20vpnc Candidate
- M 932zzu8wmqom
- Z7zgekh Twt7vm
- Z32lhpuy Fghgm
- Pfizer Vaccine Trial Bets On Early Win Against Coronavirus Documents Show Reuters
Find, Read, And Discover Pfizer Vaccine Safety Data, Such Us:
- Coverage Trumenba Meningococcal Group B Vaccine Safety Info
- U5xmqszazhv0lm
- Pfizer Could File For Vaccine Clearance Next Month
- Cthorsbe0ouikm
- Pfizer Biontech Publish Encouraging Interim Phase I Ii Data For Covid 19 Vaccine Construct
If you re looking for Sanofi Pasteur Flu Vaccine 2020 Lot Numbers you've arrived at the right location. We ve got 100 graphics about sanofi pasteur flu vaccine 2020 lot numbers including pictures, pictures, photos, wallpapers, and much more. In these page, we additionally provide number of images out there. Such as png, jpg, animated gifs, pic art, logo, black and white, transparent, etc.
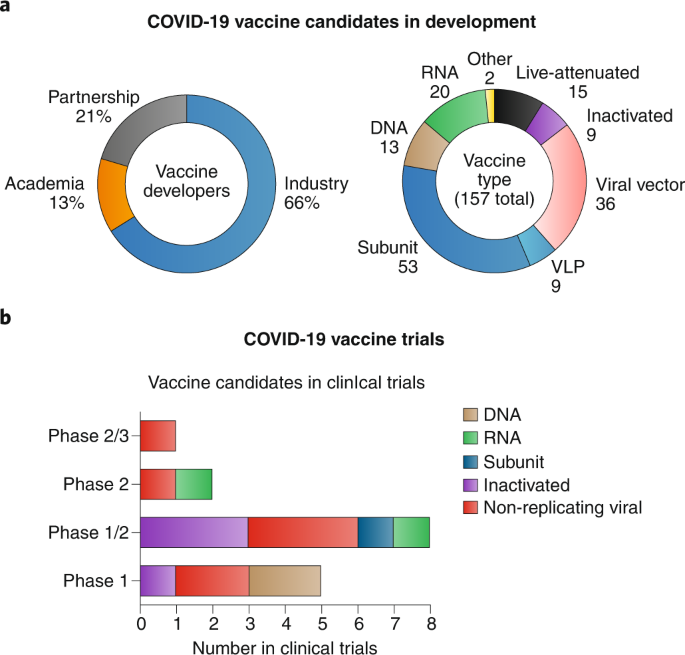
Covid 19 Vaccine Development And A Potential Nanomaterial Path Forward Nature Nanotechnology Sanofi Pasteur Flu Vaccine 2020 Lot Numbers
Phase 1 trial of two vaccine candidates in younger and older adults added to earlier interim safety and immunogenicity data regarding bnt162b1 in.

Sanofi pasteur flu vaccine 2020 lot numbers. The drug maker pfizer announced on monday that an early analysis of its coronavirus vaccine trial suggested the vaccine was robustly effective in preventing covid 19 a promising development as. Pfizer and biontech are continuing to accumulate safety data and currently estimate that a median of two months of safety data following the second and final dose of the vaccine candidate the amount of safety data specified by the fda in its guidance for potential emergency use authorization will be available by the third week of. Pfizer says an early peek at its vaccine data suggests the shots may be 90 per cent effective at preventing covid 19 indicating the company is on track later this month to file an emergency use.
Pfizer plans to ask the food and drug administration for emergency authorization of the two dose vaccine later this month after it has collected the recommended two months of safety data. As cases of coronavirus in the us. Pfizer executives expressed measured optimism tuesday over the prospect of providing a coronavirus vaccine in 2020 even as they signaled key data on the vaccine would not be released before the us.
Pfizer vaccine found 90 effective against covid 19 shows early data. We look forward to sharing additional efficacy and safety data generated from thousands of participants in the coming. The safety and immunogenicity data from this us.
Pfizer said it expects efficacy and safety data will be available in november and if it is it will apply for emergency use authorization.

Pfizer And Biontech Dose First Participants In The U S As Part Of Global Covid 19 Mrna Vaccine Development Program Pfizer Sanofi Pasteur Flu Vaccine 2020 Lot Numbers
More From Sanofi Pasteur Flu Vaccine 2020 Lot Numbers
- Covid Vaccine Trials
- Influenza Vaccine Flu Vaccine Consent Form Template
- Russian Vaccine Name For Covid
- Indonesia Vaccine Schedule
- Covid Vaccine Name
Incoming Search Terms:
- Pfizer Biontech Publish Encouraging Interim Phase I Ii Data For Covid 19 Vaccine Construct Covid Vaccine Name,
- Sqxtbgyn8rap M Covid Vaccine Name,
- Pfizer And Biontech Dose First Participants In The U S As Part Of Global Covid 19 Mrna Vaccine Development Program Pfizer Covid Vaccine Name,
- Gii Iawnjh4e8m Covid Vaccine Name,
- Abc News Live Pfizer Announces Initial Phase 3 Data Showing Its Vaccine Is Likely Effective More Safety Data Needed Before Fda Authorization Facebook Covid Vaccine Name,
- Pfizer Vaccine Trial Bets On Early Win Against Coronavirus Documents Show Reuters Covid Vaccine Name,






:strip_exif(true):strip_icc(true):no_upscale(true):quality(65)/cloudfront-us-east-1.images.arcpublishing.com/gmg/KBV4KVAJVZDPFDCUPBZOBCIDWM.jpg)