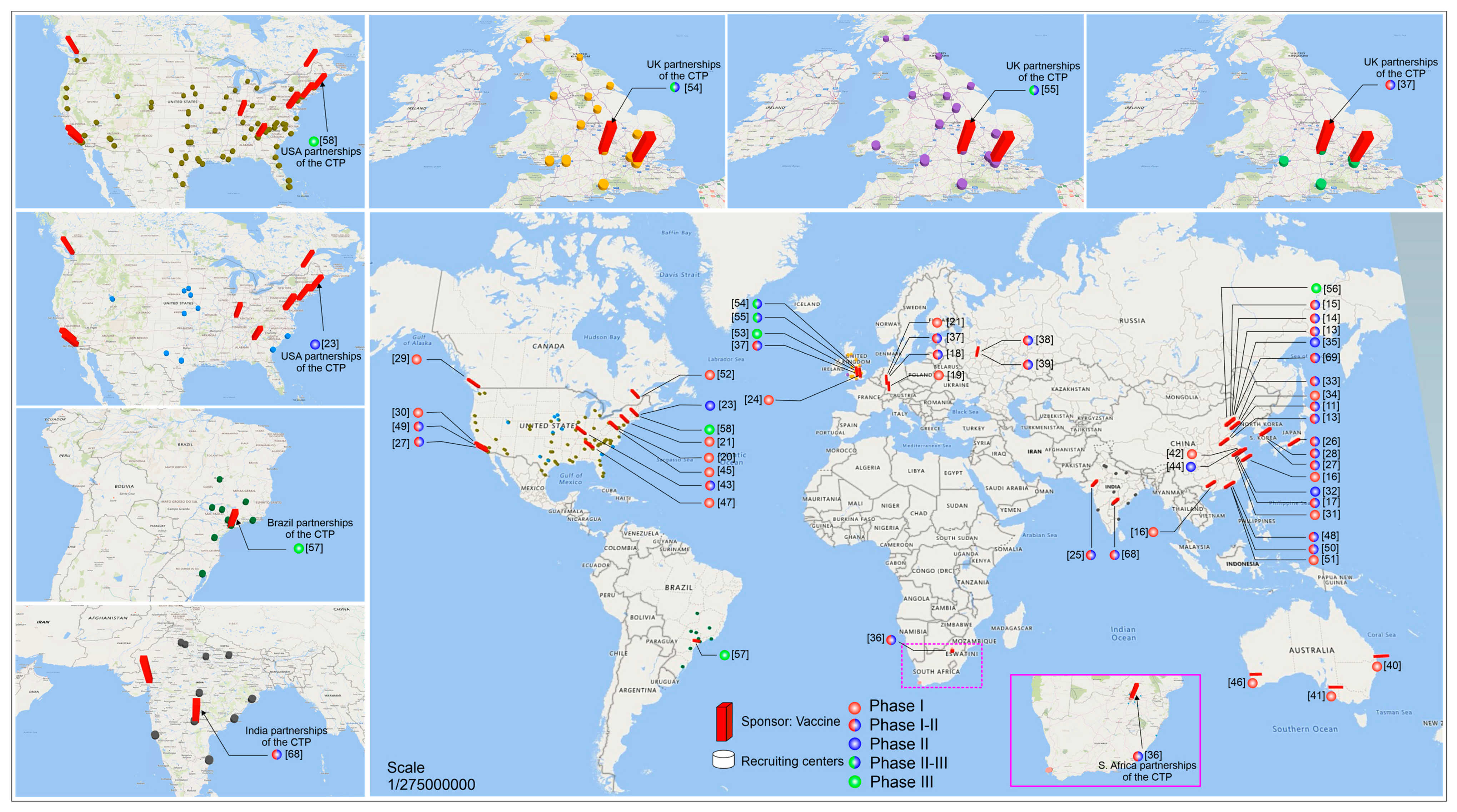
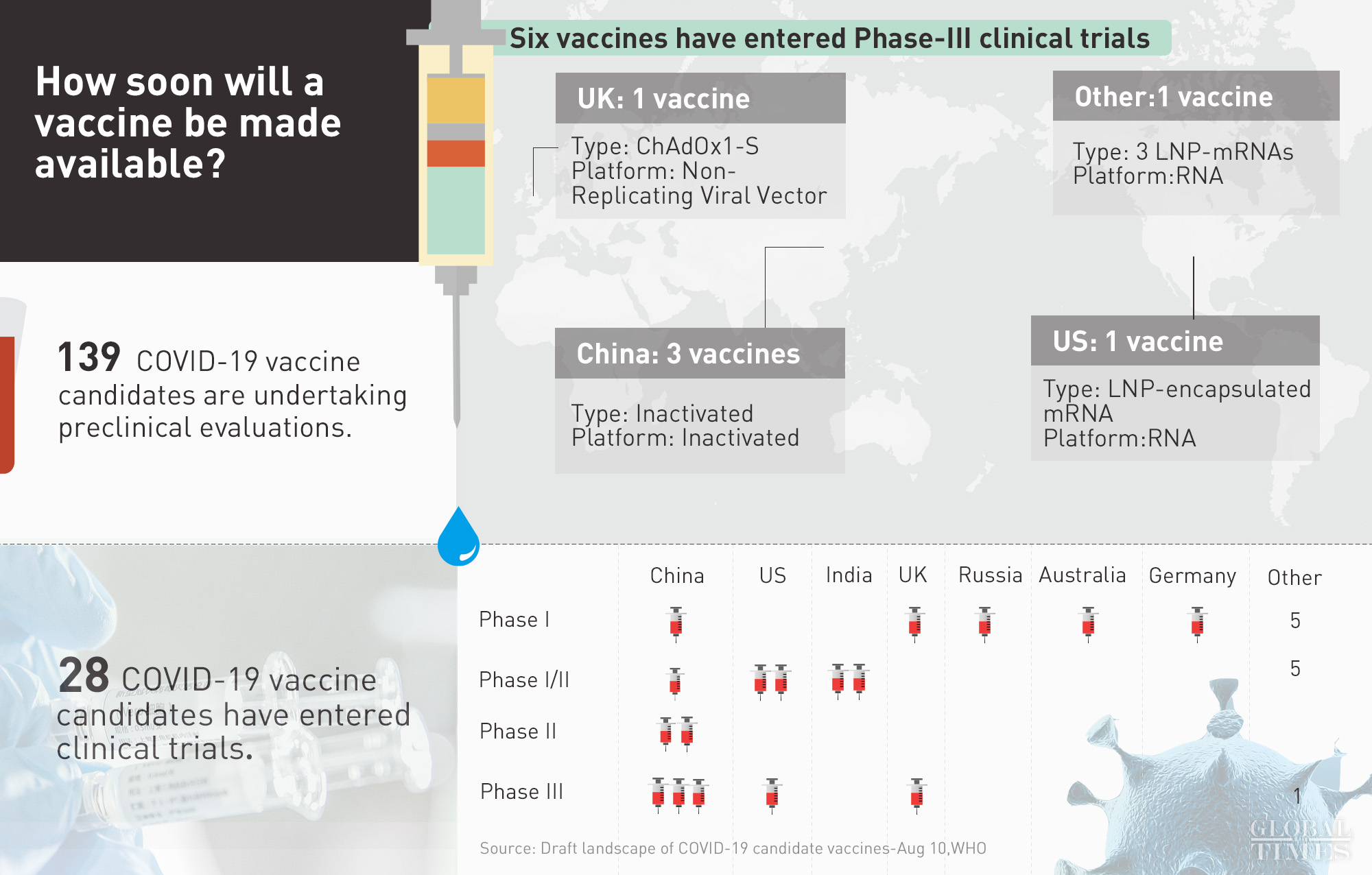
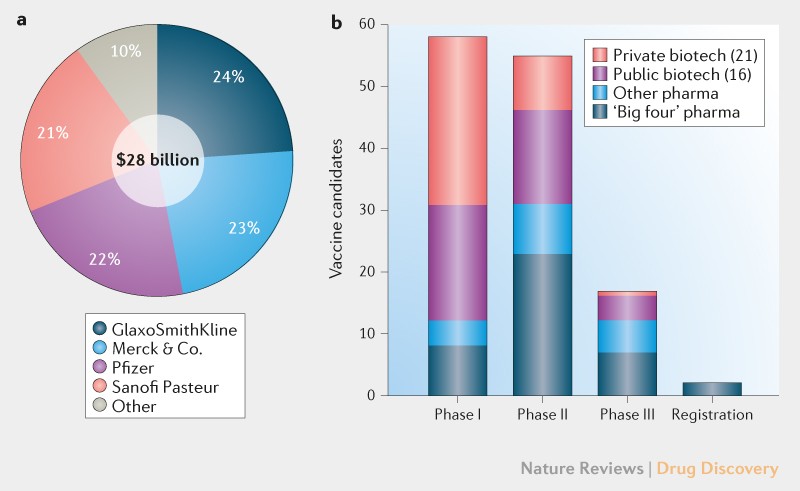
Pfizer Covid Vaccine Phase 3 Clinicaltrialsgov, Inilah 8 Kandidat Vaksin Covid 19 Yang Siap Diproduksi Massal Info Farmasi Terkini Berbasis Ilmiah Dan Praktis
Pfizer covid vaccine phase 3 clinicaltrialsgov Indeed lately is being hunted by users around us, perhaps one of you personally. Individuals are now accustomed to using the internet in gadgets to see image and video data for inspiration, and according to the title of the post I will talk about about Pfizer Covid Vaccine Phase 3 Clinicaltrialsgov.
- Brazil S Sao Paulo Likely To Start Covid 19 Immunization In December World The Jakarta Post
- Infectious Disease Vaccines
- Pfizer And Biontech Choose Lead Mrna Vaccine Candidate Against Covid 19 And Commence Pivotal Phase 2 3 Global Study Amcham Belgium
- Human Trial For Coronavirus Vaccine Launched By Moderna Enters Phase 3 Abc News
- The Astrazeneca Phase 3 Covid 19 Vaccine Study Unprecedented Compression Of Timeline Of Est Start Date To Est Primary Completion Date
- Understanding Coronavirus Clinical Studies Covid 19 Prevention Network
Find, Read, And Discover Pfizer Covid Vaccine Phase 3 Clinicaltrialsgov, Such Us:
- China Covid 19 Vaccine Could Be Ready By Year End
- Safety Tolerability And Immunogenicity Of A Recombinant Adenovirus Type 5 Vectored Covid 19 Vaccine A Dose Escalation Open Label Non Randomised First In Human Trial The Lancet
- Indonesia Chinese Vaccine Trial Volunteer Gets Virus
- Covid 19 Vaccine Frontrunners The Scientist Magazine
- 7 Covid 19 Vaccine Candidates Nearly Complete Trials Task Force Engteco News Tempo Co
If you re searching for Covid Vaccine Australia Update you've come to the right location. We ve got 100 images about covid vaccine australia update including pictures, pictures, photos, wallpapers, and much more. In such webpage, we also provide number of graphics available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
Our science find a pfizer trial.

Covid vaccine australia update. Nysepfe today announced the initiation of four phase 3 clinical trials within its current pipeline of investigational. Pfizer and biontech announced monday that their vaccine candidate against covid 19 has shown promising preliminary results in phase 3 clinical trials. An expanded cohort and efficacy part.
Meet the experts. Pfizer and biontech nasdaqbntx are on the cusp of producing phase 3 data that could show their coronavirus vaccine bnt162 protects participants from developing covid 19 better than placebo. And a respiratory syncytial virus vaccine candidate in pregnant women pfizer inc.
The statement was released. Coronavirus disease covid 19 scientific resources. A pentavalent meningococcal vaccine candidate in adolescents.
To identify preferred vaccine candidates and dose levels. The covid 19 pandemic has caused major disruption to healthcare systems with significant socioeconomic impacts. Protection in patients was achieved seven days after the second of two doses and 28 days after the first according to preliminary findings.
The vaccine is one of several that have been. The study will evaluate the safety tolerability and immunogenicity of 2 different sars cov 2 rna vaccine candidates against covid 19 and the efficacy of 1 candidate. A vaccine jointly developed by pfizer and biontech was 90 percent effective in preventing covid 19 infections in ongoing phase 3 trials the companies announced monday.
The news delivered via a press release marks the first positive data to come from a late stage covid 19 vaccine trial. A vaccine jointly developed by pfizer and biontech was 90 percent effective in preventing covid 19 infections in ongoing phase 3 trials the companies announced monday. A safe and effective vaccine for covid 19 prevention would have significant public health impact.
In long awaited good news monday drugmaker pfizer announced that its vaccine candidate created with german biotechnology company biontech se was 90 percent effective in preventing covid 19 in phase 3 clinical trials. Currently there are no specific treatments available against covid 19 and accelerated vaccine development is urgently needed. The statement was released as.
A vaccine jointly developed by pfizer and biontech was 90 effective in preventing covid 19 infections in ongoing phase 3 trials the companies announced monday.
More From Covid Vaccine Australia Update
- Vaccine Update For Covid 19 Philippines
- Eva Longoria Hair Desperate Housewives
- Down Syndrome Dog Lab
- Vaccine Latest Update
- Apple Stock Price Prediction
Incoming Search Terms:
- Pfizer And Biontech Covid 19 Vaccine Candidate Enters Phase Ii Iii Trials Apple Stock Price Prediction,
- Inilah 8 Kandidat Vaksin Covid 19 Yang Siap Diproduksi Massal Info Farmasi Terkini Berbasis Ilmiah Dan Praktis Apple Stock Price Prediction,
- The Next Triggers In Covid 19 Vaccine Development Evaluate Apple Stock Price Prediction,
- Inilah 8 Kandidat Vaksin Covid 19 Yang Siap Diproduksi Massal Info Farmasi Terkini Berbasis Ilmiah Dan Praktis Apple Stock Price Prediction,
- Gloeenqgmk7v4m Apple Stock Price Prediction,
- Db9g C7fkh7u M Apple Stock Price Prediction,