Pfizer Covid Vaccine One Or Two Shots, The Vaccines That Could Stop Covid 19 Politico
Pfizer covid vaccine one or two shots Indeed lately is being sought by consumers around us, maybe one of you. Individuals now are accustomed to using the internet in gadgets to view video and image data for inspiration, and according to the title of the article I will discuss about Pfizer Covid Vaccine One Or Two Shots.
- Forget October Warp Speed Chief Says First Covid Shot Data From Pfizer And Moderna Won T Appear Till Later This Year Fiercepharma
- J J Covid 19 Vaccine Candidate Protects Monkeys After Single Dose Fiercebiotech
- Pfizer Biontech Get 1 95 Billion Covid 19 Vaccine Order From U S Government Wsj
- When Will There Be A Coronavirus Vaccine Time
- Pfizer Begins Human Trials Of Possible Coronavirus Vaccine The New York Times
- A Comprehensive Covid 19 Vaccine Plan Center For American Progress
Find, Read, And Discover Pfizer Covid Vaccine One Or Two Shots, Such Us:
- Biontech And Pfizer S Covid 19 Vaccine Trial Yields Positive Results Financial Times
- Pfizer S Covid 19 Vaccine Proves 90 Effective In Latest Trials Fox Business
- Yls9cq1mfqnn6m
- Coronavirus Vaccine The Story Of The Race To Solve The Covid Conundrum Euronews
- Doi3vpubp6ae M
If you are searching for Stocks App Icon Aesthetic Black And White you've arrived at the right location. We ve got 100 graphics about stocks app icon aesthetic black and white adding pictures, photos, pictures, backgrounds, and much more. In such webpage, we additionally provide variety of images out there. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.
Pfizer May Apply For Emergency Use Of Covid 19 Vaccine By Late November Fox Business Stocks App Icon Aesthetic Black And White

Four Covid 19 Vaccine Candidates Induce Immune Responses In Preliminary Studies But Questions Remain Stocks App Icon Aesthetic Black And White
Pfizer was one of 15 life sciences companies that partnered with the bill melinda gates foundation in march to speed up the development and delivery of covid 19 vaccines diagnostics and treatments.
Stocks app icon aesthetic black and white. Astrazeneca is expected to start. Pfizers chief executive has said that it could have 30 to 40 million doses of the vaccine before the end of the year enough for 15 to 20 million people to get an initial shot and a booster. The vaccine will be offered in a two.
Courtesy of university of maryland school of medicine via ap. Mondays announcement doesnt mean a vaccine is imminent. Covid 19 vaccine trials have been slow to recruit black and latino people and that could delay a vaccine sanofi hasnt made announcements about whether their vaccine will be in one or two doses.
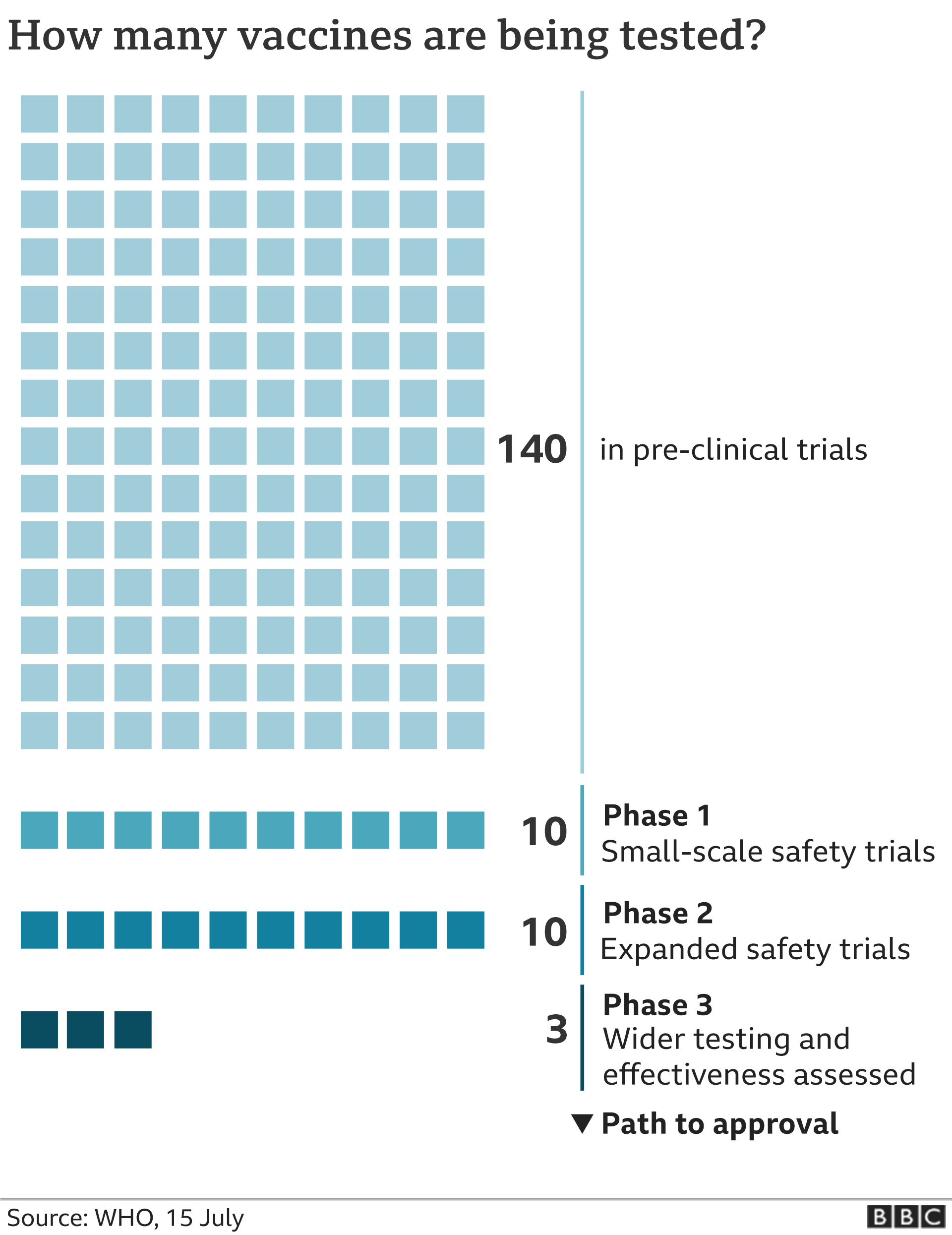
In july pfizer announced the launch of large scale phase 23 trials. 9 2020 pfizer said an early peek at its vaccine data suggests the shots may be 90 effective at preventing covid 19. Many established vaccines also require back to back doses to be most effective including the shots for measles and varicella.
Pfizer says an early peek at its vaccine data suggests the shots may be 90 effective at preventing covid 19 indicating the company is on track later this month to file an emergency use application with us. The 30000 volunteers in each of the trials are getting two doses with moderna spacing their shots out 28 days apart and pfizer spacing theirs out by 21 days. Pfizer and biontechs coronavirus vaccine inched a step closer toward approval on monday with the company announcing 90 efficacy in phase 3 clinical trial.
Interestingly bnt162b2 is one of the first covid 19 vaccines to be tested in adolescents 12 18 years of age.
Coronavirus Covid 19 Vaccine India Latest Update Oxford S Covishield In India By Year End Pfizer Shot Shows Promise Stocks App Icon Aesthetic Black And White
More From Stocks App Icon Aesthetic Black And White
- Down Syndrome Awareness Day 7
- Day Carrier Vs Vaccine Carrier
- Downton Abbey Cast Season 2 Episode 2
- Pfizer Covid Vaccine Latest News
- Whooping Cough Vaccine Side Effects Adults
Incoming Search Terms:
- Gljzasm94gxlgm Whooping Cough Vaccine Side Effects Adults,
- Coronavirus Vaccine Latest Update News Covid 19 Vaccine Current Status Here Are The 9 Top Contenders For Coronavirus Vaccines Whooping Cough Vaccine Side Effects Adults,
- Tn9p7toeshpjbm Whooping Cough Vaccine Side Effects Adults,
- Global Race To Buy Coronavirus Vaccine What You Need To Know Business Economy And Finance News From A German Perspective Dw 20 08 2020 Whooping Cough Vaccine Side Effects Adults,
- Greece Msf Uses Humanitarian Mechanism For First Time In Europe Due To High Price Of Pneumonia Vaccine Medecins Sans Frontieres Access Campaign Whooping Cough Vaccine Side Effects Adults,
- Wnallxnx8p53sm Whooping Cough Vaccine Side Effects Adults,