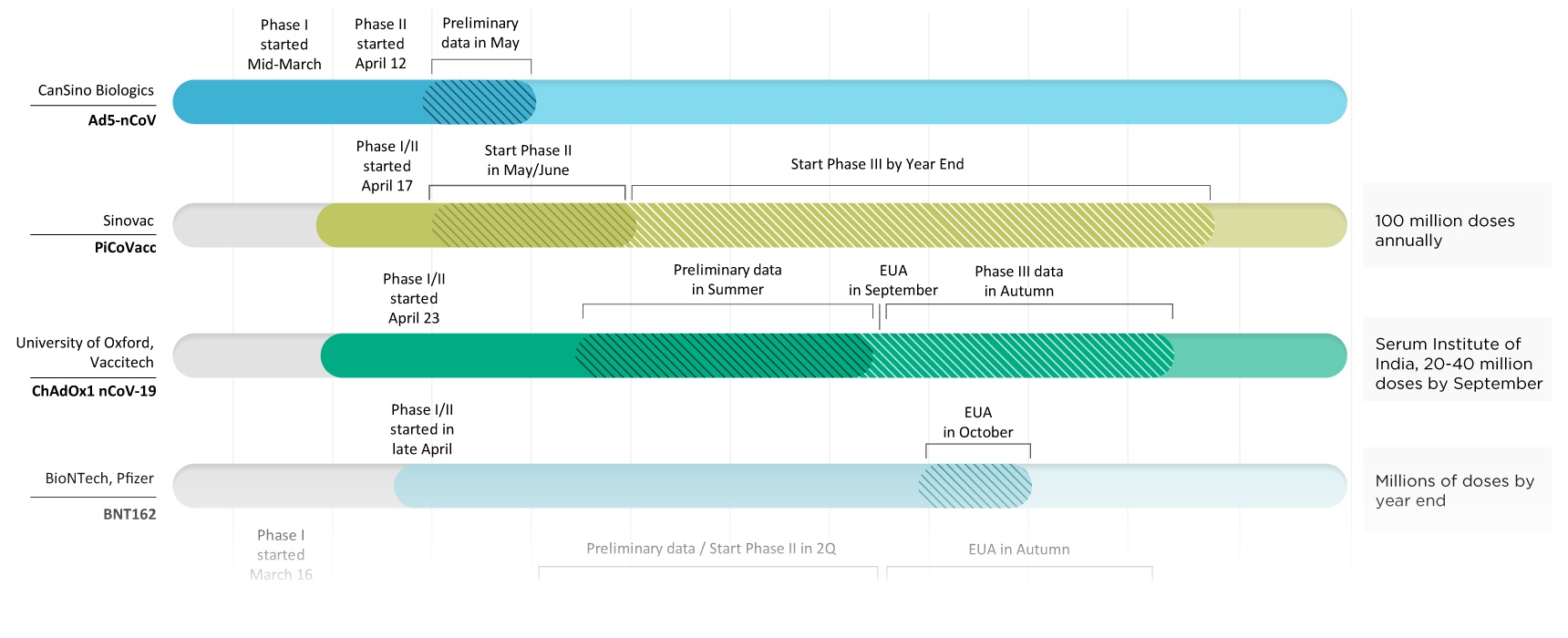
Pfizer Covid Vaccine Eua, Phe Gov Public Health Emergency Gov Barda Jpeo For Chemical Biological Radiological And Nuclear Defense And Pfizer Are Taking An Exciting Step To Make Covid19 Vaccine Available At Warpspeed Large Scale Production And
Pfizer covid vaccine eua Indeed lately has been hunted by users around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view image and video information for inspiration, and according to the name of the post I will talk about about Pfizer Covid Vaccine Eua.
- Race For Coronavirus Vaccine Accelerates As Pfizer Says U S Testing To Begin Next Week Wsj
- Pfizer Biontech See Stock Hope Soar As They Say Covid 19 Vax 90 Effective Plot November Eua Fiercebiotech
- Eric Topol على تويتر We Sent An Open Letter To Pfizer Ceo Albertbourla And The Company Bod About Their Phase 3 Vaccine Trial And How We Expect The Safety And Efficacy To
- Pfizer May Seek Us Green Light To Use Covid Vaccine In Late Nov Germany Al Jazeera
- Cx4ci9zcqrgdpm
- Is Key Safety Data At Risk In Race For Covid Vaccine
Find, Read, And Discover Pfizer Covid Vaccine Eua, Such Us:
- Biocentury End Of The Beginning For Covid 19 Vaccines
- Uk Enters Supply Agreement For Pfizer And Biontech S Covid 19 Vaccine
- Us Panel Puts The Spotlight On Pivotal Covid Vaccine Readouts Evaluate
- Pfizer Biontech S Coronavirus Vaccine Effective More Than 90
- Spotlight Pfizer Biontech Announce Covid 19 Vaccine Candidate Over 90 Percent Effective Xinhua English News Cn
If you are looking for Astrazeneca Vaccine Covid 19 September you've arrived at the ideal place. We ve got 100 images about astrazeneca vaccine covid 19 september including pictures, photos, photographs, backgrounds, and more. In such webpage, we also have number of images available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, transparent, etc.

Coronavirus Usa What Phase Is The Pfizer And Moderna Vaccine In As Com Astrazeneca Vaccine Covid 19 September
The drugmaker pfizer said it will not apply for emergency use authorization for its covid 19 vaccine candidate until.

Astrazeneca vaccine covid 19 september. As pfizer biontech covid 19 vaccine showed more than 90 per cent efficacy in preventing coronavirus the preliminary results have now paved the way for the companies to seek an emergency use authorisation from the regulators if further research shows the shot is also safe. Pfizerbiontech covid 19 vaccine 90 effective us. Pfizer biontechs covid 19 vaccine is 90 effective eua filing likely by late november.
The pfizer chief said friday that although the company may determine whether the vaccine is effective or not by the end of this month its ongoing collection of safety data will push back its filing for an eua beyond october. Pfizer ceo says dont expect covid 19 vaccine eua until after election. Which is the amount of safety data specified by the fda in its guidance for potential emergency use.
Pfizer will not seek emergency use authorization eua for its covid 19 vaccine until the end of november even if the readout from a phase iii study expected later this month is positive. October 28th 2020 at 1048 am. The pharmaceuticals giant pfizer announced monday that its coronavirus vaccine is more than 90 percent effective according to any early analysis of the data from a clinical trial involving 44000.
This morning pfizer chief executive officer albert bourla posted an open letter on the companys website explaining three key metrics the experimental vaccine for the novel coronavirus must meet before. Pfizer will not be ready to submit an emergency use authorization eua application for its covid 19 vaccine candidate until late in november ceo albert bourla has announced despite his previous projections of possibly filing for an eua this month. Pfizers ceo albert bourla has published a statement on the pharmaceutical companys efforts on developing a covid 19.
Coronavirus vaccine candidate maker pfizer was expected to release early efficacy results for phase 3 in mid october ahead of the presidential election. Eua application expected this month.
Pfizer Biontech Covid 19 Vaccine May Be Ready For Fda Submission By Late November Drug Topics Astrazeneca Vaccine Covid 19 September
More From Astrazeneca Vaccine Covid 19 September
- Covid Vaccine Failed
- Three Legged Downward Dog Yoga Pose
- Stocks Vs Bonds Vs Mutual Funds
- Astrazeneca Vaccine Trial
- Vaccine Us
Incoming Search Terms:
- Yj1ybwjfkppom Vaccine Us,
- Xconomy Pfizer S Bourla There S Coronavirus Vaccine Pressure But It S Not Political Vaccine Us,
- Ec Concludes Talks With Pfizer Biontech For Covid 19 Vaccine Supply Vaccine Us,
- Fda Issues Covid 19 Vaccine Guidance As White House Reportedly Relents Medcity News Vaccine Us,
- Coronavirus Trump Administration Unveils Cvs Walgreens Vaccine Deal Vaccine Us,
- Us Vaccine Program Chief Backs Stricter Rules For Emergency Use Of Covid 19 Shot World The Jakarta Post Vaccine Us,