Moderna Covid Vaccine Trial Protocol, Coronavirus Vaccine Trials Won T Tell Us If They Save Lives Prevent Serious Illness Or Stop Transmission Here S Why
Moderna covid vaccine trial protocol Indeed lately has been hunted by consumers around us, perhaps one of you. Individuals now are accustomed to using the internet in gadgets to see image and video information for inspiration, and according to the title of the article I will discuss about Moderna Covid Vaccine Trial Protocol.
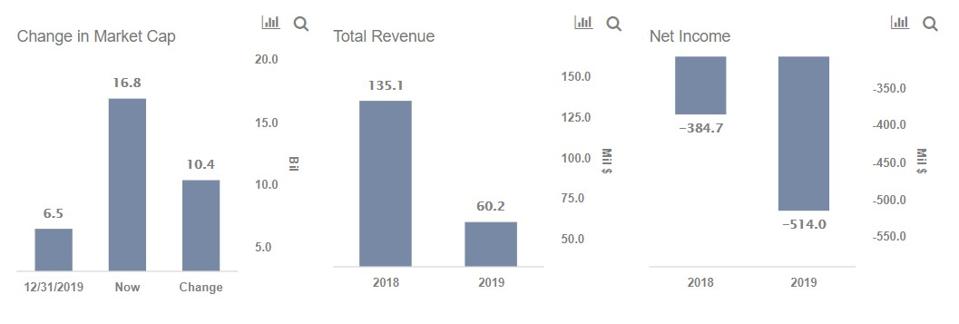
- Covid 19 Vaccine Study Results Could Come Late October Moderna Ceo Says Wsj
- Moderna Shares Covid 19 Vaccine Trial Blueprints Will Others Follow World The Jakarta Post
- Moderna Finalizing Protocol For Ph 3 Study Of Covid 19 Vaccine
- Top Adviser Steps Aside From Fda Covid 19 Vaccine Reviews Over Potential Conflict
- Nih Clinical Trial Of Investigational Vaccine For Covid 19 Begins National Institutes Of Health Nih
- Fda Clears Moderna Phase Ii Trial For Covid 19 Vaccine Candidate
Find, Read, And Discover Moderna Covid Vaccine Trial Protocol, Such Us:
- What To Expect As Moderna S Covid 19 Vaccine Commences Phase 2 Trials
- Fda Grants Fast Track Designation To Moderna S Covid 19 Vaccine
- Some Volunteers Want To Be Infected With Coronavirus To Help Find A Vaccine But It Isn T That Simple Fivethirtyeight
- Ukslvdxtlw5fxm
- Chinese Vaccine Maker To Offer Shots To Testing Nations First
If you re searching for Influenza Vaccine Consent Form 2020 Pdf you've reached the ideal location. We ve got 100 graphics about influenza vaccine consent form 2020 pdf including images, photos, pictures, wallpapers, and much more. In such page, we also provide number of graphics out there. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.
J J Kicks Off Study Of Single Shot Covid 19 Vaccine In 60 000 Volunteers World The Jakarta Post Influenza Vaccine Consent Form 2020 Pdf

Phase Iii Clinical Trial Of Covid 19 Vaccine Begins Technology Networks Influenza Vaccine Consent Form 2020 Pdf
View a listing of clinical trial sites.

Influenza vaccine consent form 2020 pdf. Moderna and pfizer are on the human clinical trial stage of their respective covid 19 vaccine candidates. Moderna publishes phase iii covid 19 vaccine study protocol as trial enrolls more than 80 of participants the company said at its rd day that the phase iii cove trial of mrna 1273 had. Numerous bets are ultimately an.
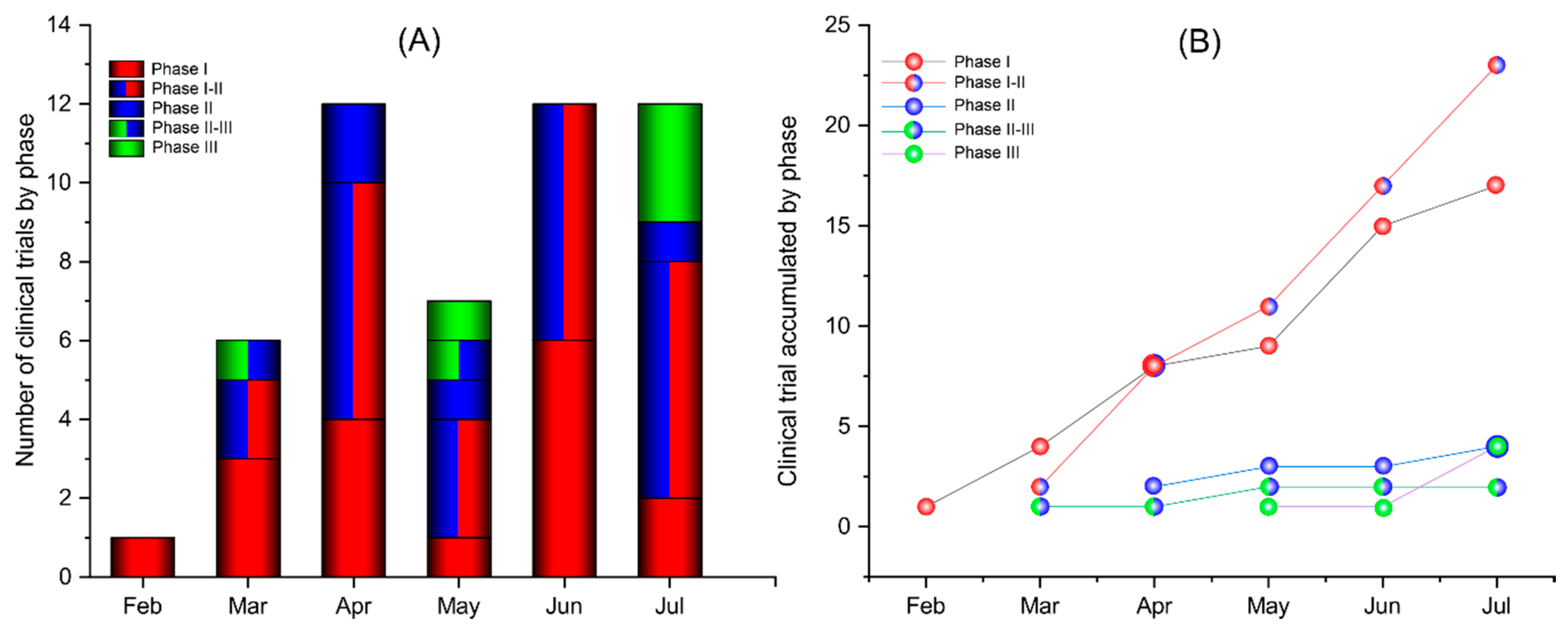
In september following months of campaigning for greater openness12 four manufacturers made their full study protocols publicly available3456 the publications create a rare opportunity for real time transparency. A rare opportunity for public scrutiny of these key trials the ongoing phase iii trials for covid 19 vaccines are some of the most consequential randomised trials ever done. The vaccine was in fact given to thousands of people.
The study is designed to primarily evaluate the efficacy safety and immunogenicity of mrna 1273 to prevent covid 19 for up to 2 years after the second dose of mrna 1273. The purpose of this study is to test modernas vaccine candidate that may prevent illness after exposure to the sars cov 2 virus which causes covid 19. A former version of the article stated that 53 people received a vaccination for interim analysis in the moderna trial.
Modernas vaccine candidate mrna 1273 is nearing the finish line in its push to enroll. By enrolling in this study participants are contributing to a potential solution that could help to solve this global health crisis. Moderna has finalised the protocol for the phase iii clinical trial of its covid 19 vaccine candidate mrna 1273 based on feedback from the us food and drug administration fda.
The trial is set to be performed in partnership with the us national institutes of health nihs national institute of allergy and infectious diseases niaid. Moderna releases key details on how its running covid 19 vaccine trial by nicoletta lanese staff writer 17 september 2020 the moderna vaccine is among the frontrunners to potentially be approved. 100 ug of mrna 1273 vaccine or a placebo control in a 11 randomization ratio.
Assignment will be stratified by age and health risk. All participants will be assessed for efficacy and safety endpoints and.
More From Influenza Vaccine Consent Form 2020 Pdf
- Hepatitis A Vaccine Consent Form
- Pfizer Covid 19 Vaccine Storage Temperature
- Covid Vaccine Australia Trials
- Zona Vaccine
- Pfizer Vaccine How Effective
Incoming Search Terms:
- Covid 19 Vaccines To Enter Late Stage Trial By End Of July Fauci Says Cgtn Pfizer Vaccine How Effective,
- Moderna Phase Iii Covid 19 Vaccine Study To Enroll 30 000 Participants Starting Next Month Medcity News Pfizer Vaccine How Effective,
- Some Volunteers Want To Be Infected With Coronavirus To Help Find A Vaccine But It Isn T That Simple Fivethirtyeight Pfizer Vaccine How Effective,
- Byj Av A4 Wvpm Pfizer Vaccine How Effective,
- Moderna Stocks Drop Over 8 On Vaccine Trial Delay Teletrader Com Pfizer Vaccine How Effective,
- Towards Effective Covid 19 Vaccines Updates Perspectives And Challenges Review Pfizer Vaccine How Effective,