Moderna Covid Vaccine Phase 3 Protocol, Phase 3 Trial For Moderna Covid 19 Vaccine Begins Amid Us Summer Surge Cidrap
Moderna covid vaccine phase 3 protocol Indeed lately has been sought by consumers around us, maybe one of you. People now are accustomed to using the internet in gadgets to view video and image data for inspiration, and according to the name of this article I will discuss about Moderna Covid Vaccine Phase 3 Protocol.
- Researchers Publish Encouraging Early Data On Covid 19 Vaccine Nih Director S Blog
- Safety Tolerability And Immunogenicity Of A Recombinant Adenovirus Type 5 Vectored Covid 19 Vaccine A Dose Escalation Open Label Non Randomised First In Human Trial The Lancet
- Immunogenicity And Safety Of A Recombinant Adenovirus Type 5 Vectored Covid 19 Vaccine In Healthy Adults Aged 18 Years Or Older A Randomised Double Blind Placebo Controlled Phase 2 Trial The Lancet
- Moderna Initiates Phase Iii Trial Of Covid 19 Vaccine Candidate
- Moderna Begins Dosing In Phase 3 Study Of Mrna Covid 19 Vaccine Candidate Covid 19 Hospimedica Com
- Moderna Eyes Early Summer Start For Phase 3 Covid 19 Vaccine Trial Fiercebiotech
Find, Read, And Discover Moderna Covid Vaccine Phase 3 Protocol, Such Us:
- Covid Pandemic Why Indonesia Became Testing Ground For China S Covid 19 Vaccine Bloomberg
- Covid 19 Vaccine Progress Uc Health To Launch Phase 3 Clinical Trial Uc Health
- Coronavirus Vaccines Get A Biotech Boost
- Moderna Coronavirus Vaccine Starts Phase 3 Trial What To Expect Business Insider
- Moderna Delay A Snapshot Of Squabbles With U S Scientists Over Covid 19 Vaccine Trials Reuters Fiercebiotech
If you are searching for Pfizer Covid Trial News you've reached the ideal location. We ve got 100 graphics about pfizer covid trial news including images, pictures, photos, wallpapers, and more. In such web page, we additionally have number of images out there. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.
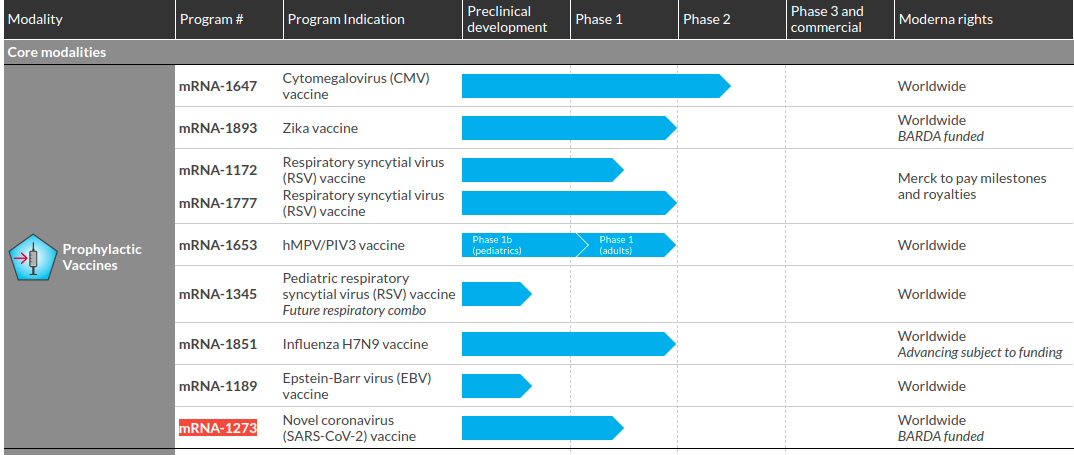
The vaccine known as mrna 1273 was co developed by the cambridge massachusetts based biotechnology company moderna inc and the national institute of allergy and infectious diseases.
Pfizer covid trial news. Two dose regimen of chikungunya antibody mrna 1944 demonstrates the platforms ability for safe repeat. The late stage trial will include 30000 participants and is expected. Food and drug administration fda guidance on clinical trial design for covid 19 vaccine studies.
The biotech firms leading candidate has just entered phase three clinical trials in the us. The randomized placebo controlled trial is expected to include approximately 30000 participants in the united states testing an mrna 1273 dosage of 100 ug. The mrna 1273 vaccine is being developed to prevent covid 19 the disease resulting from severe acute respiratory syndrome coronavirus sars cov 2 infection.
A phase 3 randomized stratified observer blind placebo controlled study to evaluate the efficacy safety and immunogenicity of mrna 1273 sars cov 2 vaccine in adults aged 18 years and older protocol number. A phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 covid 19 in adults has begun. The trial is set to be performed in partnership with the us national institutes of health nihs national institute of allergy and infectious diseases niaid.
When at least 151 cases of covid 19 in total occur in both vaccinated and unvaccinated people moderna will be able to tell whether its vaccine is at least 60 effective the times reported. Moderna to enter seasonal flu business given the high medical need for more effective flu vaccine. Moderna publishes phase iii covid 19 vaccine study protocol as trial enrolls more than 80 of participants the company said at its rd day that the phase iii cove trial of mrna 1273 had.
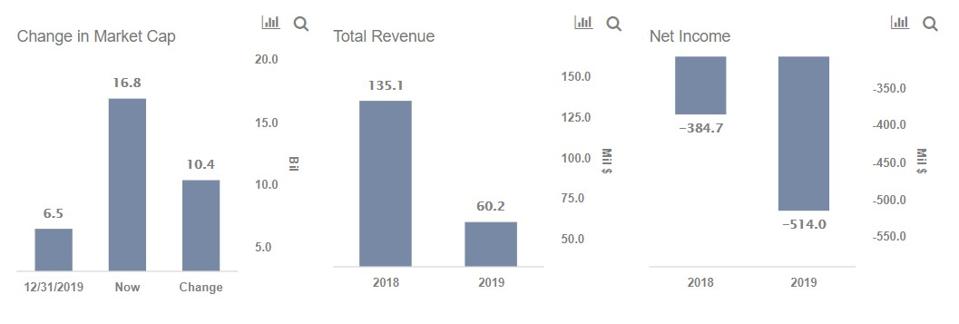
Phase 3 cove study of covid vaccine candidate mrna 1273 has enrolled 25296 participants to date. Moderna has finalised the protocol for the phase iii clinical trial of its covid 19 vaccine candidate mrna 1273 based on feedback from the us food and drug administration fda. Phase 3 protocol now available online.
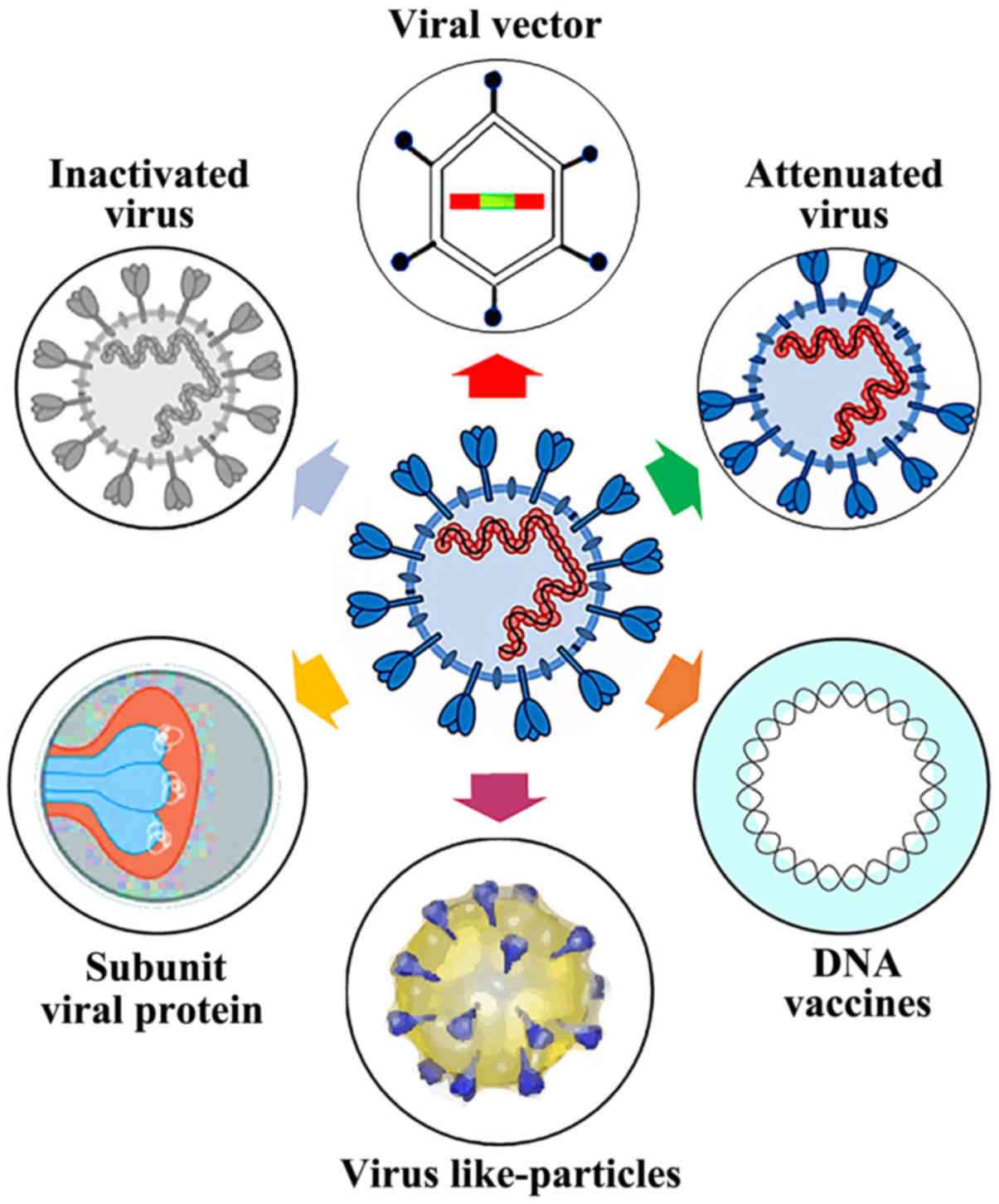
Find out why this vaccine technology is promisingbut not without its skeptics. The phase 3 study protocol follows the us.
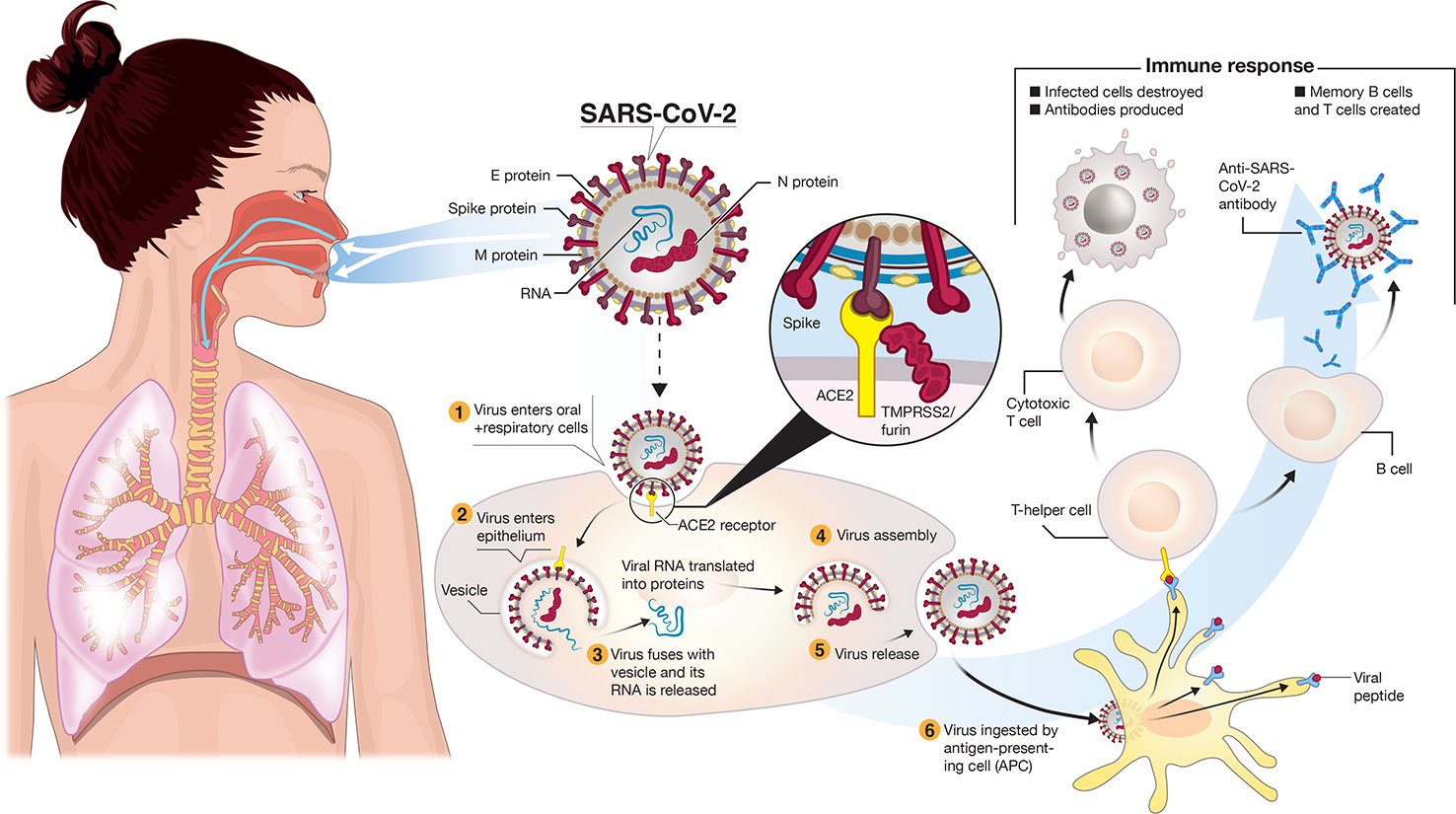
Frontiers A Snapshot Of The Global Race For Vaccines Targeting Sars Cov 2 And The Covid 19 Pandemic Pharmacology Pfizer Covid Trial News
More From Pfizer Covid Trial News
- Injection Anti Rabies Vaccine
- Hepatitis B Vaccine Clip Art
- Indonesia Vaccine Schedule
- Pfizer Covid Vaccine Open Letter
- Pfizer Stock Undervalued
Incoming Search Terms:
- Canada Contracts With Novavax For 76 Million Vaccine Doses For 2021 World The Jakarta Post Pfizer Stock Undervalued,
- Moderna Shares Covid 19 Vaccine Trial Blueprints Pfizer Follows Pfizer Stock Undervalued,
- Xconomy Moderna Pfizer Protocols May Make Covid Vaccines Hard To Compare Pfizer Stock Undervalued,
- Moderna To Begin Phase 3 Of Covid 19 Vaccine Study In July Drug Discovery And Development Pfizer Stock Undervalued,
- J J Adds Chile Argentina And Peru To Latin America Covid 19 Vaccine Trials World The Jakarta Post Pfizer Stock Undervalued,
- Covid Pandemic Why Indonesia Became Testing Ground For China S Covid 19 Vaccine Bloomberg Pfizer Stock Undervalued,