Mmr Vaccine Name Canada, Vaccinating In The Age Of Apathy Measles Vaccination In Canada 1963 1998 Cmaj
Mmr vaccine name canada Indeed recently is being hunted by users around us, perhaps one of you. People are now accustomed to using the net in gadgets to view video and image data for inspiration, and according to the name of the post I will talk about about Mmr Vaccine Name Canada.
- Do Children Who Receive An Early Dose Of Mmr Vaccine During A Measles Outbreak Return For Their Regularly Scheduled Dose A Retrospective Population Based Study Bmj Open
- Facebook Pledges To Fight Vaccine Misinformation Amid Mounting Criticism Ctv News
- Get Vaccinated For Measles Canadian Cancer Survivor Network
- Mmr Vaccine Wikipedia
- Who Are The Anti Vaxxers Here S What We Know And How They Got There In The First Place National Post
- Canadian Immunization Guide Chapter On Influenza And Statement On Seasonal Influenza Vaccine For 2020 2021 Canada Ca
Find, Read, And Discover Mmr Vaccine Name Canada, Such Us:
- Pdf Largest Measles Epidemic In North America In A Decade Quebec Canada 2011 Contribution Of Susceptibility Serendipity And Superspreading Events
- Influenza Vaccination Coverage Across Ethnic Groups In Canada Abstract Europe Pmc
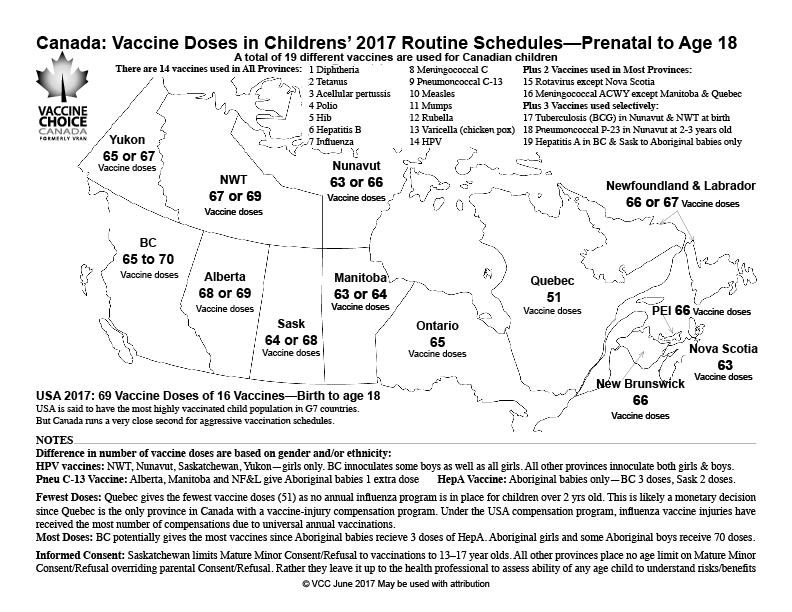
- Shot In The Dark On Vaccinations For Measles And Other Diseases Data Gaps Leave Canadians Guessing The Globe And Mail
- Vaccinating In The Age Of Apathy Measles Vaccination In Canada 1963 1998 Cmaj
- 2
If you re looking for Vaccine Management you've come to the ideal place. We have 100 graphics about vaccine management adding images, photos, photographs, wallpapers, and more. In such web page, we also provide variety of graphics available. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.
1983 mmr measles mumps rubella immunization program introduced for all infants.
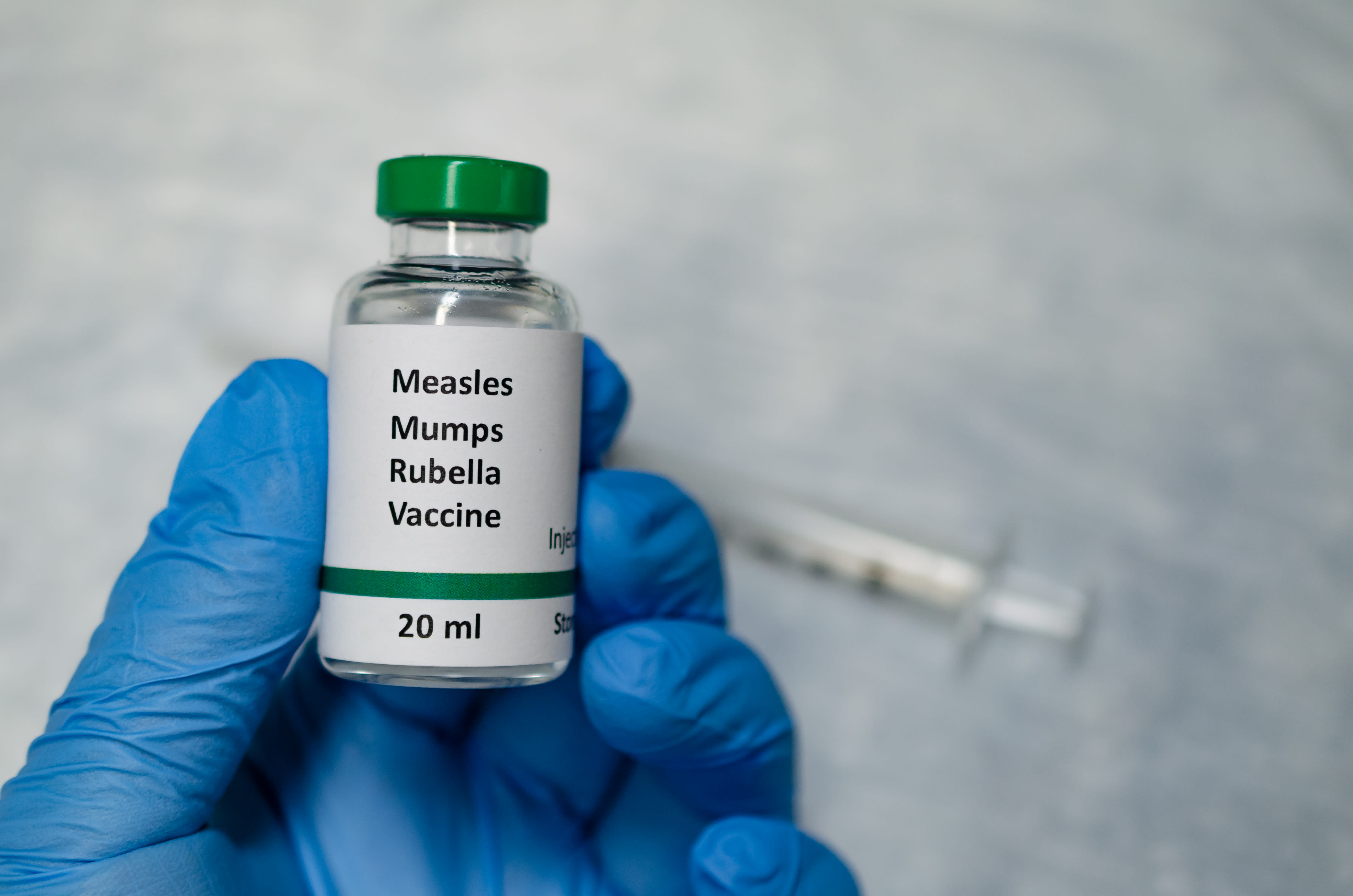
Vaccine management. Sanofi was established in 1973 as a subsidiary of elf aquitane. Monitoring the quality of vaccines authorized for sale in canada. Doses may not be required depending upon age of child or vaccine used or both refer to the relevant vaccine specific chapter in part 4 and.
The measles mumps rubella and varicella mmrv vaccine also protects against these diseases. The headquarters of this top. Authorizing only vaccines that are safe effective and of high quality and whose benefits outweigh the risks.
The mmr vaccine pluserix known as trivirix in canada uses the urabe mumps strain. Mmr vaccine or human immunoglobulin ig may be used for measles post exposure immunization in susceptible persons. How canada buys stores and handles vaccines and how supply gaps or delays are addressed and prevented vaccine safety surveillance and reporting how vaccines are regulated and approved report an adverse event following immunization vaccine safety publications.
M m r ii pdf 11 pages external icon the food and drug administration fda approved this vaccine in 1971 for use in people 12 months of age and older. Mmr ii is supplied freeze dried lyophilized and contains live viruses. Sanofi is another of the top vaccine manufacturers in the world specialized in research and development of highly effective vaccination for babies.
The measles mumps and rubella vaccine is made from live measles mumps and rubella viruses that have been weakened to the point where they will not cause disease. Rubella cases went from approximately 5300 per year between 1971 and 1982 to fewer than 30 cases per year between 1988 and 1994. Recommended immunization schedule children less than 7 years of age not previously immunized as infants.
The measles mumps and rubella vaccine belongs to a group of medication known as vaccines. The abbreviations on this table column 3 were standardized jointly by staff of the centers for disease control and prevention acip work groups the editor of the morbidity and mortality weekly report mmwr the editor of epidemiology and prevention of vaccine preventable diseases the pink book acip members and liaison organizations to the acipthese abbreviations are intended to. Measles vaccine is available as measles mumps rubella mmr or measles mumps rubella varicella mmrv vaccine.
1983 pneumococcal polysaccharide vaccine is approved for use in canada. Before injection it is reconstituted with the solvent provided. 1988 hib haemophilus influenzae vaccine introduced in.
It is used to prevent infection with measles rubeola mumps and rubella german measles. Subsequently sanofi later merged with aventis in 2004 and changed its name to sanofi in may 2011. It is no longer used in the uk or canada but it remains.
For children at risk due to underlying medical conditions refer to table 4 for additional recommendations for immunization. Evaluating a vaccine for its safety efficacy and quality based on scientific and clinical evidence. Health canada regulates vaccines by.
More From Vaccine Management
- Covid Vaccine Production Stopped
- Neon Stocks App Icons
- Down Arrow Png Gif
- Pfizer Vaccine News Today
- Russian Vaccine Name Sputnik
Incoming Search Terms:
- Measles And Measles Prevention Let S Talk Science Russian Vaccine Name Sputnik,
- Global Trial To Test Whether Mmr Vaccine Protects Front Line Health Care Workers Against Covid 19 Washington University School Of Medicine In St Louis Russian Vaccine Name Sputnik,
- Vaccination And Your Child Caring For Kids Russian Vaccine Name Sputnik,
- Varicella Vaccine Wikipedia Russian Vaccine Name Sputnik,
- Abortion Opponents Protest Covid 19 Vaccines Use Of Fetal Cells Science Aaas Russian Vaccine Name Sputnik,
- Nhs Warns Of Growing Public Health Timebomb As Half A Million Uk Children Miss Measles Vaccination Leicestershire Live Russian Vaccine Name Sputnik,



/arc-anglerfish-tgam-prod-tgam.s3.amazonaws.com/public/7WGT6U6LX5GDPGWNIYBSHMNUOM)


