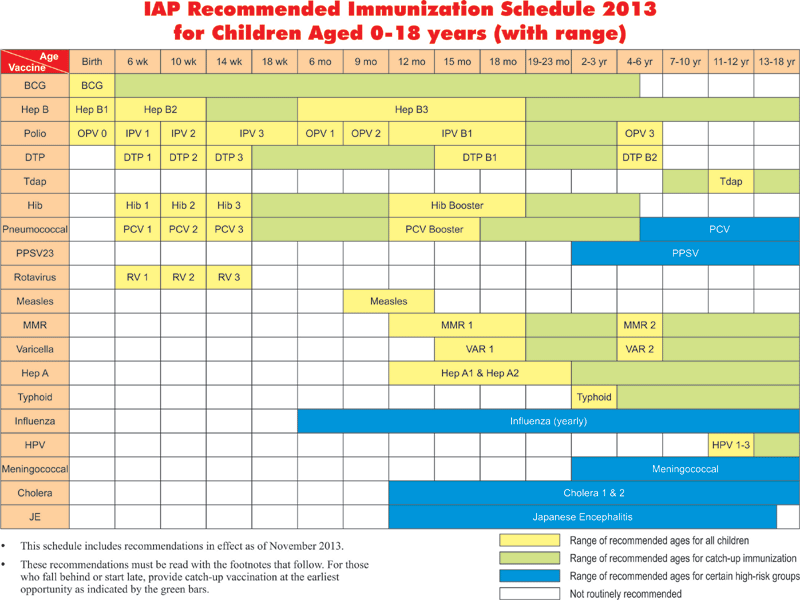
Japanese Encephalitis Vaccine Dose Schedule, Imojev Full Prescribing Information Dosage Side Effects Mims Malaysia
Japanese encephalitis vaccine dose schedule Indeed lately has been sought by users around us, perhaps one of you. People are now accustomed to using the internet in gadgets to view image and video information for inspiration, and according to the name of the article I will talk about about Japanese Encephalitis Vaccine Dose Schedule.
- Who Japanese Encephalitis Vaccine Inactivated
- Side Effects Reported By Recipients Of Biken Japanese Encephalitis Download Table
- Https Www Michigan Gov Documents Mdch Japanese Encepha03012010 313310 7 Pdf
- Imojev Powder For Injection Nps Medicinewise
- 2
- Immunizations In Autoimmune Inflammatory Rheumatic Disease In Adults Uptodate
Find, Read, And Discover Japanese Encephalitis Vaccine Dose Schedule, Such Us:
- Ixiaro Japanese Encephalitis Virus Vaccine Schedule Booster Dose
- October 2018 Acip Meeting Japanese Encephalitis Youtube
- A Replication Defective Japanese Encephalitis Virus Jev Vaccine Candidate With Ns1 Deletion Confers Dual Protection Against Jev And West Nile Virus In Mice Npj Vaccines
- Bharat Biotech Pharma Jenvac P Bhogilal And Sons Id 7555738812
- Japanese Encephalitis Vaccine Information Statement
If you are looking for Covid Vaccine Update Ireland you've arrived at the right location. We have 104 graphics about covid vaccine update ireland adding pictures, photos, photographs, wallpapers, and much more. In these page, we additionally have variety of graphics out there. Such as png, jpg, animated gifs, pic art, logo, black and white, transparent, etc.
Complete primary immunization series at least 1 week prior to potential exposure to jev.
Covid vaccine update ireland. 18 through 65 years. Is pregnantpregnant women should usually not get je vaccine. In the accelerated schedule group 99 of adults were seroprotected compared with 100 of adults in the standard schedule group.
The primary immunization schedule is 2 doses administered on days 0 and 28. Two types of japanese encephalitis vaccines are provided in travel health centres subject to supply availability inactivated vaccine this vaccine is registered for use in travellers aged 2 months and above. 2 doses 05 ml im administered 28 days apart.
To administer a 025 ml dose health. 2 doses 05 ml administered either 7 or 28 days apart 66 years. An accelerated schedule is possible 3 doses on day 0 day 7 and day 14 but this is likely to result in lower antibody levels than the standard schedule.
Has had an allergic reaction after a previous dose of je vaccine or has any severe life threatening allergies. Inactivated vero cell culture derived japanese encephalitis je vaccine manufactured as ixiaro is the only je vaccine licensed and available in the united states. The accelerated primary series was noninferior to the conventional dosing schedule.
Vaccine recommended for adults at increased risk of japanese encephalitis virus jev during travel to asia. The inactivated mouse brain derived imb vaccine is now commonly replaced by cell culture based vaccines. No information is available concerning the interchangeability of vaccines.
Vaccination should be postponed in the event of severe. This vaccine was approved in march 2009 for use in people aged 17 years and older and in may 2013 for use in children 2 months through 16 years of age. Japanese encephalitis and japanese encephalitis vaccine 2 shorter term eg 1 month tr avelers with an increased risk of je based on planned travel duration season location activities and accommodations.
Tell your vaccine provider if the person getting the vaccine. Do not administer to patients with history of an allergic reaction to a previous injection of japanese encephalitis vaccine. The primary vaccination dose and schedule for ixiaro varies by age.
Japanese encephalitis vaccine information statement.
More From Covid Vaccine Update Ireland
- Vaccine News Coronavirus
- Vaccine Schedule
- Mmr Vaccine Price In India
- Corona Vaccine Logo
- Down Syndrome Ultrasound Vs Normal 11 Weeks
Incoming Search Terms:
- Japanese Encephalitis Vaccine Information Statement Down Syndrome Ultrasound Vs Normal 11 Weeks,
- Japanese Encephalitis Vaccination In The Philippines A Cost Effectiveness Analysis Comparing Alternative Delivery Strategies Sciencedirect Down Syndrome Ultrasound Vs Normal 11 Weeks,
- Coverage Missed Opportunity For Japanese Encephalitis Vaccine Gorakhpur Division Uttar Pradesh India 2015 Implications For Japanese Encephalitis Control Murhekar Mv Oak C Ranjan P Kanagasabai K Shinde S Pandey Ak Mittal Down Syndrome Ultrasound Vs Normal 11 Weeks,
- Infants In Sarawak Given Japanese Encephalitis Vaccine Dr Jamilah Borneo Post Online Down Syndrome Ultrasound Vs Normal 11 Weeks,
- Jenvac Japanese Encephalitis Vaccine In India Bharat Biotech Down Syndrome Ultrasound Vs Normal 11 Weeks,
- Side Effects Reported By Recipients Of Biken Japanese Encephalitis Download Table Down Syndrome Ultrasound Vs Normal 11 Weeks,