Influenza Vaccine 202021, Https Www Healthvermont Gov Sites Default Files Documents Pdf Id Iz Infohcpmain Vibflu 08 2020 Pdf
Influenza vaccine 202021 Indeed lately has been sought by consumers around us, perhaps one of you. People now are accustomed to using the internet in gadgets to view video and image data for inspiration, and according to the title of the article I will discuss about Influenza Vaccine 202021.
- 2020 2021 Influenza Vaccine Codes Pricing And Recommendations Aapc Knowledge Center
- Seqirus Begins Shipping 2020 21 Influenza Vaccines To U S Market Cdr Chain Drug Review
- Influenza Vaccine For 2020 2021 The Medical Letter Inc
- Come Get Your Free Pei Flu Vaccine Or Not Ethical And Social Commentary On Life In Pei
- Delivering 2020 21 Flu Immunisation During The Coronavirus Pandemic Nursing Times
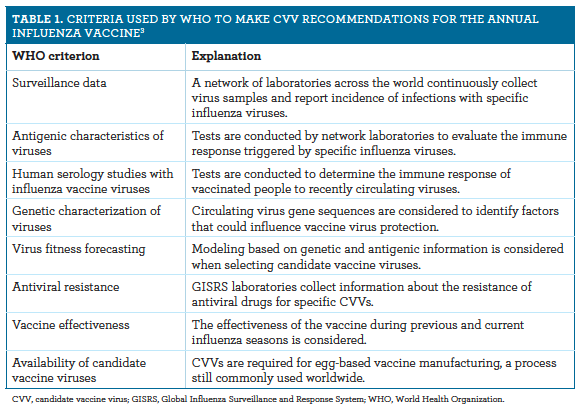
- An Update On The Who And Acip 2020 2021 Influenza Vaccine Recommendations
Find, Read, And Discover Influenza Vaccine 202021, Such Us:
- Https Www Michigan Gov Documents Flu Riv Ql Final 700762 7 Pdf
- Flu Vaccination Programme 2020 21 Hsc Public Health Agency
- Flu Vaccinations 2020 21
- Influenza Australian Government Department Of Health
- Https Www Hopkinsmedicine Org Hse Occupational Health Flu Faq 2020 Pdf
If you re looking for Covid Vaccine Questions Remained you've come to the ideal location. We ve got 104 graphics about covid vaccine questions remained adding pictures, photos, photographs, backgrounds, and more. In these webpage, we also have number of graphics out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
Rx Item Afluria Pfs 60mcg 5ml Quad Flu Vaccine 20 21 By Sequiris 36 Months Covid Vaccine Questions Remained
Package inserts for us licensed vaccines external icon.

Covid vaccine questions remained. Vaccines europe 28 jul 2020. It is recommended that quadrivalent vaccines for use in the 2020 2021 northern hemisphere influenza season contain the following. Prevention and control of seasonal influenza with vaccines.
Influenza vaccines for the 2020 2021 influenza season 5. The centre for health protection chp of the department of health dh today august 24 announced that the vaccination subsidy scheme vss and the government vaccination programme gvp 202021 will be launched on october 8 and 22 respectively providing in phases subsidised or free seasonal influenza vaccination siv and pneumococcal vaccination pv to eligible persons. Cdc recommends annual influenza vaccination for everyone 6 months and older with any licensed age appropriate flu vaccine iiv riv4 or laiv4 with no preference expressed for any one.
Influenza vaccine ativ eg g rown t iv lent influenza vaccine surface antigeninactivated adjuvanted with mf59c1 from 65 years equal to or less than 02 micrograms per 05 ml dose 08457 451 500 sanofi pasteur vaccines trivalent influenza vaccine high dose tiv hd high dose egg grown trivalent influenza vaccine split virion. Recommendations of the advisory committee on immunization practices united states 2020 2021 influenza season has been published. For those aged 6 months through 8 years the number of doses of influenza vaccine needed for the 202021 influenza season is determined as follows.
Those who have previously received 2 total doses of trivalent or quadrivalent influenza vaccine 4 weeks apart before july 1 2020 require only 1 dose for the 202021 season. Vaccination providers should consult fda approved prescribing information for 202021 influenza vaccines for the most complete and updated information including but not limited to indications contraindications warnings and precautions. Quadrivalent influenza vaccines will contain hemagglutinin ha derived from these three viruses as well as an additional influenza b viral component from the byamagata lineage which is unchanged from that included in quadrivalent influenza vaccines used during the 201920 season.
Vaccines europe supports efforts by member states and the european commission ec to prevent simultaneous outbreaks of seasonal influenza and covid 19 which would place a considerable strain on health systemsthrough 2020 there has been an increased demand for influenza vaccines as health authorities seek to keep people at risk of influenza complications and. Influenza vaccines including for adults 65 years of age and over is available on the ministry of healths website. Recommended composition of influenza virus vaccines for use in the 2020 2021 northern hemisphere influenza season 28 february 2020.
More From Covid Vaccine Questions Remained
- Small Vaccine Carrier Box
- Tetanus Vaccine Price India
- Vaccine For Covid 19 Updates In India
- Modernas Fotos De Casas Bonitas Por Dentro
- Hepatitis B Vaccine Dose In Bangladesh
Incoming Search Terms:
- 5buer8esdgjwrm Hepatitis B Vaccine Dose In Bangladesh,
- Http Www Bccdc Ca Resource Gallery Documents Guidelines 20and 20forms Guidelines 20and 20manuals Epid Cd 20manual Chapter 202 20 20imms Part4 Influenza Eligibility Pdf Hepatitis B Vaccine Dose In Bangladesh,
- Centre For Health Protection Vaccination Subsidy Scheme General Public Hepatitis B Vaccine Dose In Bangladesh,
- Flu 2020 21 The Flu Calculator And Overview Guidance Wessex Lmcs Hepatitis B Vaccine Dose In Bangladesh,
- First Flu Related Death Reported In North Carolina For 2020 21 Flu Season Wlos Hepatitis B Vaccine Dose In Bangladesh,
- Https Www England Nhs Uk Wp Content Uploads 2020 05 National Flu Immunisation Programme 2020 2021 Pdf Hepatitis B Vaccine Dose In Bangladesh,