Hpv Vaccine Guidelines Cdc, Hpv Vaccination For Young Adults
Hpv vaccine guidelines cdc Indeed recently has been sought by users around us, perhaps one of you personally. Individuals are now accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of the article I will talk about about Hpv Vaccine Guidelines Cdc.
- Boosting Hpv Vaccination Rates A Call To Action Women S Healthcare
- Cdc Votes On Age Expansion For Hpv Vaccine Tmc News
- Who Should Get The Hpv Vaccine Ob Gyn Associates Of Marietta Obstetrics And Gynecologist
- Hpv Vaccination Recommendation
- The Hpv Vaccine Access And Use In The U S Kff
- Hpv Vaccine Safety And Effectiveness Hse Ie
Find, Read, And Discover Hpv Vaccine Guidelines Cdc, Such Us:
- Illinois State Medical Society
- Hpv For Clinicians Vaccination Schedules And Recommendations Cdc
- Hpv Vaccine Administration Human Papillomavirus Vaccination Cdc
- The Hpv Vaccine Access And Use In The U S Kff
- Vaccine Safety Musc Health Charleston Sc
If you are searching for Moderna Covid Vaccine Trial Sign Up you've come to the right location. We have 104 graphics about moderna covid vaccine trial sign up including pictures, photos, pictures, backgrounds, and more. In such page, we also provide number of graphics available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, transparent, etc.

Human Papillomavirus In 2019 An Update On Vaccines And Dosing Recommendations Consult Qd Moderna Covid Vaccine Trial Sign Up
Gardasil 9 protects against infection with.

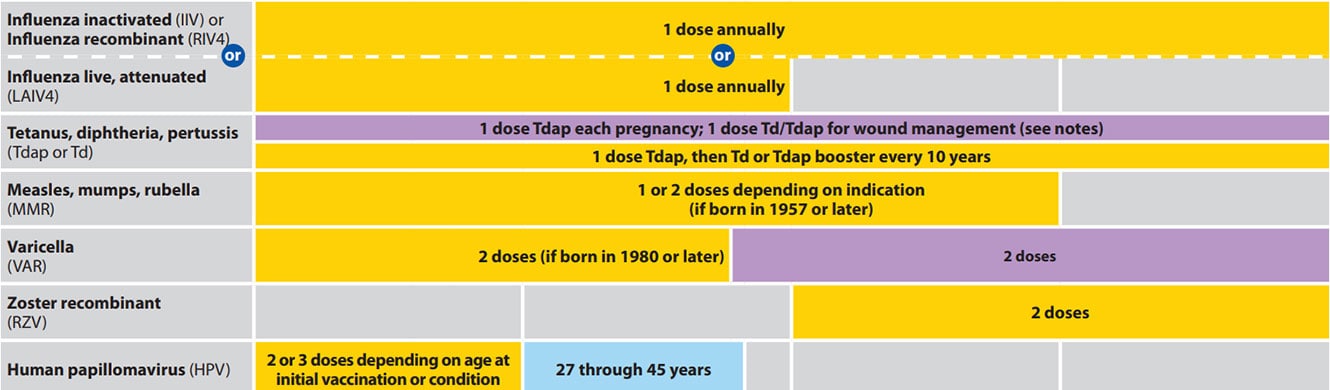
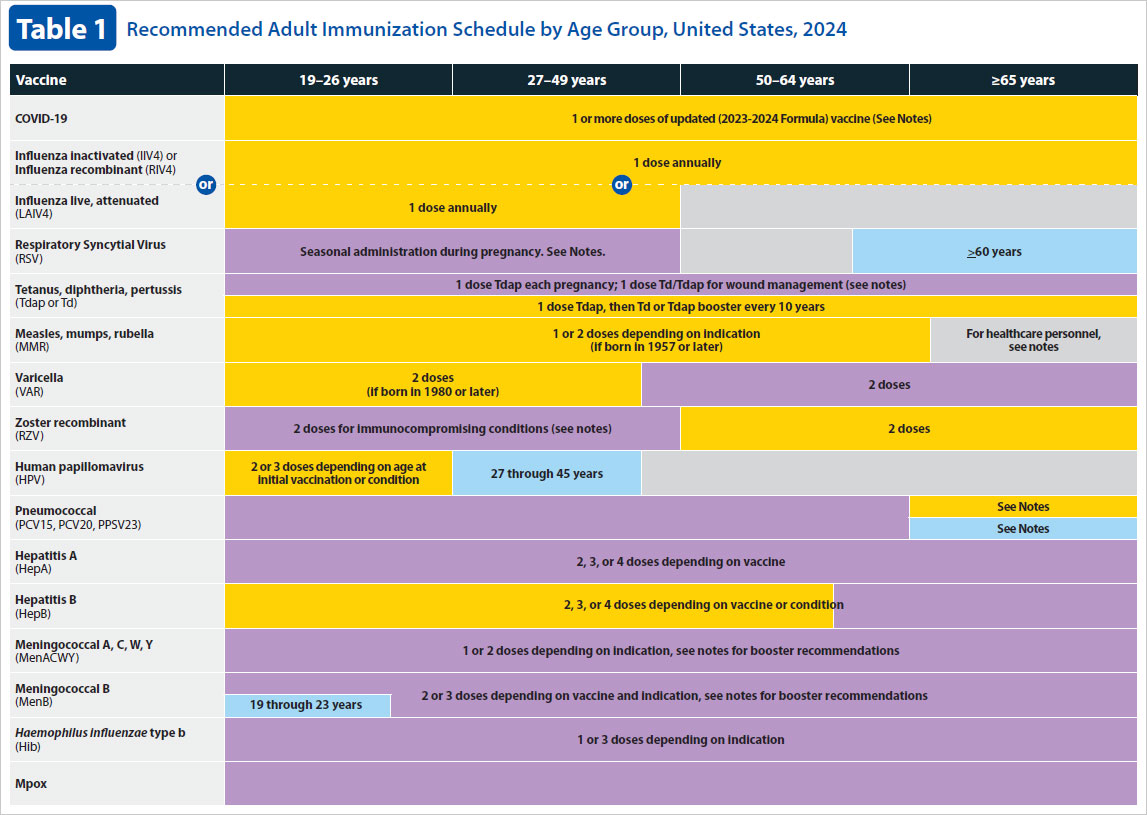
Moderna covid vaccine trial sign up. Hpv vaccination is given as a series of either two or three doses depending on age at initial. Cdc recommends 3 doses of hpv vaccine 0 12 6 month schedule for people age 9 through 26 years if they have certain immunocompromising conditions. Hpv vaccination in this age range provides less benefit as more people have already been exposed to hpv.
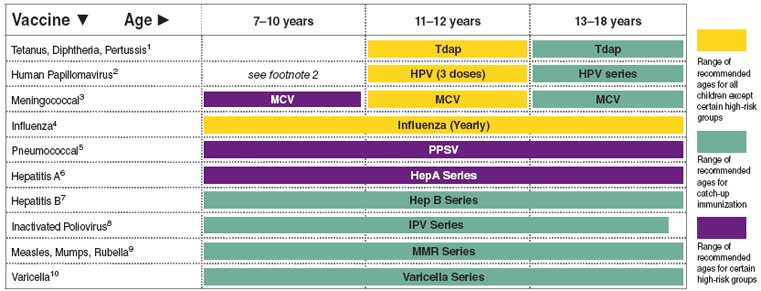
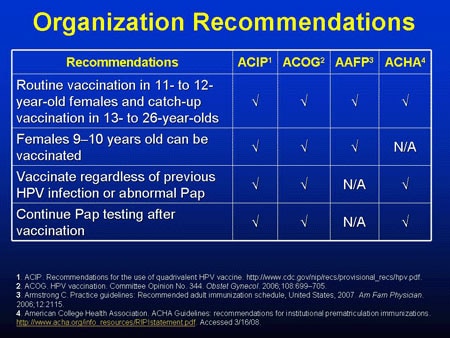
Cdc will publish more detailed hpv vaccination guidelines for health care providers parents and insurers. Vaccination can be started at age 9 acip also recommends vaccination for everyone through age 26 years if not adequately vaccinated previously. Getting your 11 12 year old child two doses of the hpv vaccine can prevent these cancers.
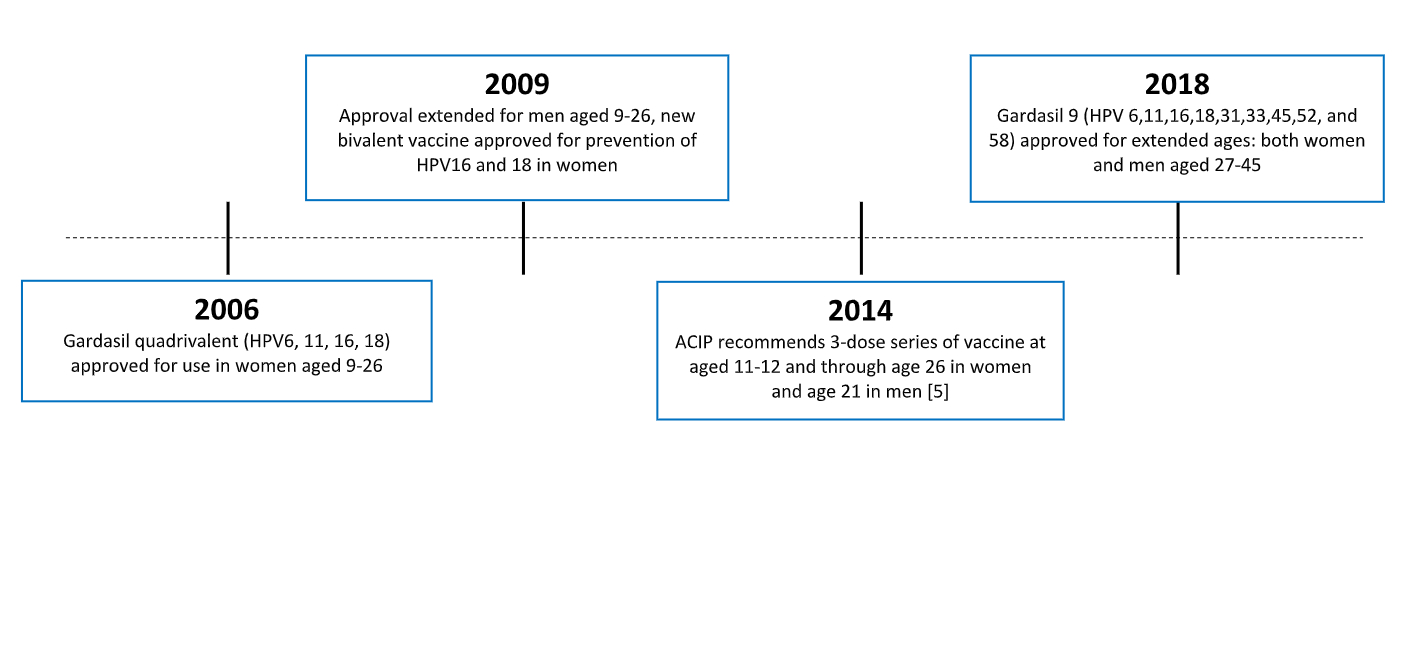
Gardasil 9 is a vaccine indicated in females 9 through 45 years of age for the prevention of cervical vulvar vaginal anal oropharyngeal and other head and neck cancers caused by human papillomavirus hpv types 16 18 31 33 45 52 and 58. Hpv vaccination is preventing cancer causing infections and precancers. Recommendations for hpv vaccination in the united states before june 26 2019 routine vaccination age 11 or 12 years vaccination can be started at age 9 years.
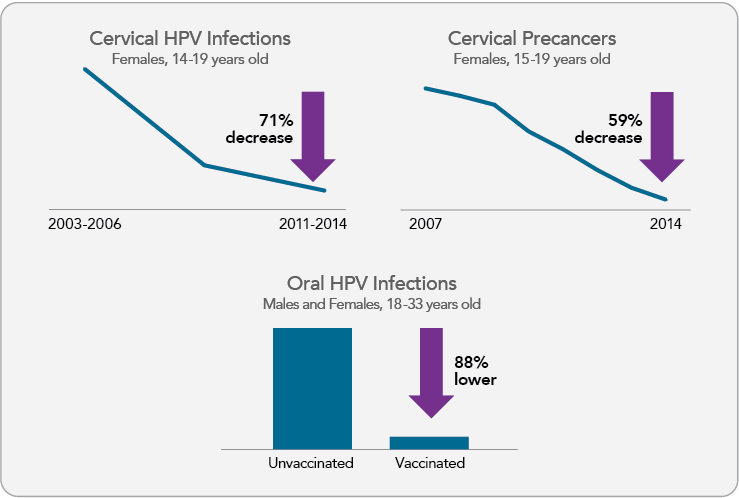
Cdc continues to monitor hpv vaccine safety and impact of the vaccination program on hpv attributable outcomes including prevalence of hpv infections anogenital warts precancers and cancers. The new recommendations followed the october 7 approval by the food and drug administration fda of a two dose regimen of the hpv vaccine gardasil 9 for boys and girls ages 9 to 14 years old. Hpv infections and cervical precancers abnormal cells on the cervix that can lead to cancer have dropped significantly since the vaccine has been in use.
Catch up hpv vaccination is now recommended for all persons through age 26 years. What are the implications for public health practice. Skip directly to site content skip directly to page options skip directly to a z link.
Cervical vulvar vaginal and anal precancerous or dysplastic. Routine recommendations for hpv vaccination of adolescents have not changed. People whose immune responses might be lower for example due to hiv infection cancer transplantation autoimmune disease or taking immunosuppressant medications should receive 3 doses to.
Indication for gardasil 9 human papillomavirus 9 valent vaccine recombinant. After reviewing new evidence cdc updated hpv vaccination recommendations for us. For adults aged 27 through 45 years.
Hpv is a common virus that can lead to certain types of cancer later in life.
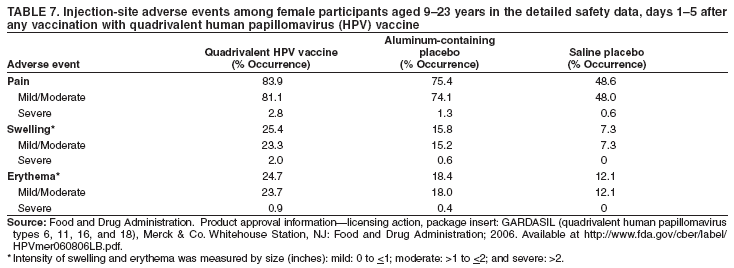
Quadrivalent Human Papillomavirus Vaccine Recommendations Of The Advisory Committee On Immunization Practices Acip Moderna Covid Vaccine Trial Sign Up
More From Moderna Covid Vaccine Trial Sign Up
- When Will We See Vaccine Kids
- Downton Abbey House Price
- Tetanus Toxoid Vaccine Price Philippines
- Frente Planos De Casas Modernas De 2 Pisos Gratis Con Medidas
- Biontech Pfizer Vaccine
Incoming Search Terms:
- Ppt Speaker Name Speaker Title Speaker Affiliation Powerpoint Presentation Id 5409233 Biontech Pfizer Vaccine,
- Quadrivalent Human Papillomavirus Vaccine Recommendations Of The Advisory Committee On Immunization Practices Acip Biontech Pfizer Vaccine,
- The Hpv Vaccine Access And Use In The U S Kff Biontech Pfizer Vaccine,
- U S Vaccine Guidelines For Flu Hpv Updated Biontech Pfizer Vaccine,
- Fda Hpv Vaccine Recommendations When Will Insurance Cover It For People Ages 27 45 Biontech Pfizer Vaccine,
- Hpv Vaccine Recommendation Cdc Panel Advises Expanding Ages Cnn Biontech Pfizer Vaccine,