Astrazeneca Covid Vaccine Trial Us, After Astrazeneca Some Quit J J Covid 19 Trial In Spain Germany News Al Jazeera
Astrazeneca covid vaccine trial us Indeed lately has been sought by users around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to see image and video information for inspiration, and according to the title of this article I will talk about about Astrazeneca Covid Vaccine Trial Us.
- Astrazeneca Resumes Us Covid 19 Vaccine Trial Novinite Com Sofia News Agency
- Coronavirus Vaccine Australia Secures Access To Oxford Astrazeneca Trial Bbc News
- New Data Offer Glimpse Of Efficacy Of Oxford Astrazeneca Covid 19 Vaccine
- Astrazeneca S Covid 19 Vaccine Reaches Phase 3 Clinical Trials In Us President Donald Trump
- Fda Expands Probe Of Astrazeneca S Covid 19 Vaccine Us Trial Still Delayed
- Fda Widens Probe Into Astrazeneca S Us Coronavirus Vaccine Trial Report Fox Business
Find, Read, And Discover Astrazeneca Covid Vaccine Trial Us, Such Us:
- Fda Expands Probe Of Astrazeneca S Covid 19 Vaccine Us Trial Still Delayed
- Astrazeneca To Resume Coronavirus Vaccine Trial In U S Axios
- Astrazeneca And Johnson Johnson To Resume Covid 19 Vaccine Trials As U S Daily Cases Top 71 000 Cbs News
- Phase Iii Trials Of Astrazeneca Covid 19 Vaccine Candidate Begins In Us Express Pharma
- 500 000 Us Children Diagnosed With Covid 19 Astrazeneca Pauses Vaccine Trial Wral Techwire
If you are looking for Corona Virus Vaccine In India you've come to the right location. We have 100 images about corona virus vaccine in india adding images, photos, photographs, wallpapers, and much more. In such web page, we additionally provide number of images available. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.

Astrazeneca Covid 19 Vaccine Trial In U S On Hold Until At Least Midweek Newsnation Now Corona Virus Vaccine In India
Astrazeneca and johnson johnson will both resume their paused coronavirus vaccine trials in the us.
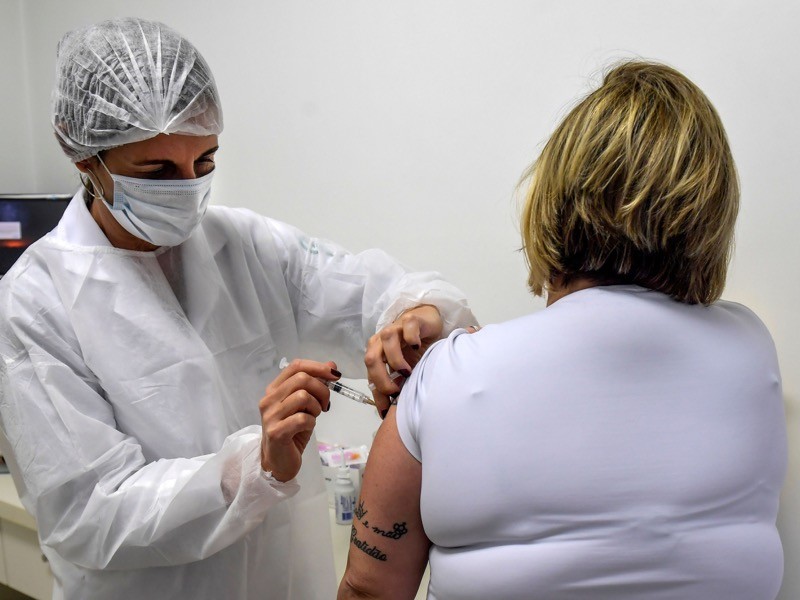
Corona virus vaccine in india. A participant in astrazeneca and oxfords covid 19 vaccine trial in brazil receives a shot. While other holds globally have been lifted the us. 1 the us trial called d8110c00001 is funded by the biomedical advanced development authority barda part of the office of the assistant secretary for preparedness and response aspr at the us.
Azd1222 development expanded into a phase iii clinical trial in the us to assess its safety efficacy and immunogenicity. The united kingdom based global biopharmaceutical company astrazeneca is leading the trial as regulatory sponsor. Astrazeneca expects results from trials of the coronavirus vaccine it is developing with oxford university by the end of the year offering hope in the fight against a rapidly resurging pandemic.
As us health authorities consider whether to allow astrazeneca to resume the clinical trial of its coronavirus vaccine crucial questions remain about neurological illnesses suffered by study. Department of health and human services hhs and the national institute of. Astrazenecas covid 19 vaccine trial remains on hold in the united states pending a us investigation into a serious side effect in the united kingdom even as other trials of the vaccine resume.
Fda authorises restart of the covid 19 azd1222 vaccine us phase iii trial. The fda reviewed all safety data from trials globally and concluded it was safe to resume the trial clinical trials for the astrazeneca oxford coronavirus vaccine azd1222 have resumed across the world with regulators in the us uk brazil south africa and japan confirming that it was safe to do so the statement added.
More From Corona Virus Vaccine In India
- Pfizer Covid Vaccine Adjuvant
- Down Syndrome Animals Cow
- Covid Vaccine Update Indian Express
- Subunit Vaccine Adjuvant
- Biontech Se Logo
Incoming Search Terms:
- Astrazeneca Covid 19 Vaccine Trial On Hold Over Safety Issue Economy News Al Jazeera Biontech Se Logo,
- Final Phase Clinical Trial Of Astrazeneca Covid 19 Vaccine Begins In Us Global Times Biontech Se Logo,
- Fda Expands Probe Of Astrazeneca S Covid 19 Vaccine Us Trial Still Delayed Biontech Se Logo,
- Astrazeneca Resumes Trials Of Covid 19 Vaccine In Britain Voice Of America English Biontech Se Logo,
- Astrazeneca Resumes Us Covid 19 Vaccine Trial Optimism Seen For J J Business Standard News Biontech Se Logo,
- Astrazeneca Johnson Johnson To Resume Covid 19 Vaccine Trials In Us Health Albanyherald Com Biontech Se Logo,