Adjuvanted Trivalent Influenza Vaccine 2020, Https Www Brightonandhoveccg Nhs Uk Wp Content Uploads Sites 5 2020 09 Flu Faq On The Vaccine Sussex Ccgs 23 09 20 Pdf
Adjuvanted trivalent influenza vaccine 2020 Indeed recently is being sought by consumers around us, maybe one of you. People are now accustomed to using the internet in gadgets to view image and video information for inspiration, and according to the title of the post I will talk about about Adjuvanted Trivalent Influenza Vaccine 2020.
- Cracking The Code Of Optimized Influenza Prevention Cell Based Influenza Vaccine Advancement
- Efficacy Immunogenicity And Safety Of An Oral Influenza Vaccine A Placebo Controlled And Active Controlled Phase 2 Human Challenge Study The Lancet Infectious Diseases
- Nhs Letter Advises On Ordering Flu Vaccines For 2020 21 Season Latest Pharmacy News Business Magazine Pharmacy Business
- Http Www Cpwy Org Doc 2896 Pdf
- Inactivated Flu Vaccine Vaccine Knowledge
- Over 65s Flu Jab Supplier On Track To Meet Agreed Times Pharmacy Network News
Find, Read, And Discover Adjuvanted Trivalent Influenza Vaccine 2020, Such Us:
- Pdf Inactivated Trivalent Influenza Vaccine Is Associated With Lower Mortality Among Covid 19 Patients In Brazil
- New Evidence On Relative Effectiveness Of Adjuvanted Seasonal Influenza Vaccine In Seniors Precision Vaccinations
- 3
- 2020 2021 Influenza Vaccine Codes Pricing And Recommendations Aapc Knowledge Center
- Pdf A Randomized Controlled Study To Evaluate The Safety And Reactogenicity Of A Novel Rvlp Based Plant Virus Nanoparticle Adjuvant Combined With Seasonal Trivalent Influenza Vaccine Following Single Immunization In Healthy Adults 18 50
If you re searching for Covid Vaccine Launch Date In Russia you've reached the perfect place. We have 104 images about covid vaccine launch date in russia including images, pictures, photos, backgrounds, and much more. In such web page, we additionally have variety of graphics available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.

New Evidence On Relative Effectiveness Of Adjuvanted Seasonal Influenza Vaccine In Seniors Precision Vaccinations Covid Vaccine Launch Date In Russia
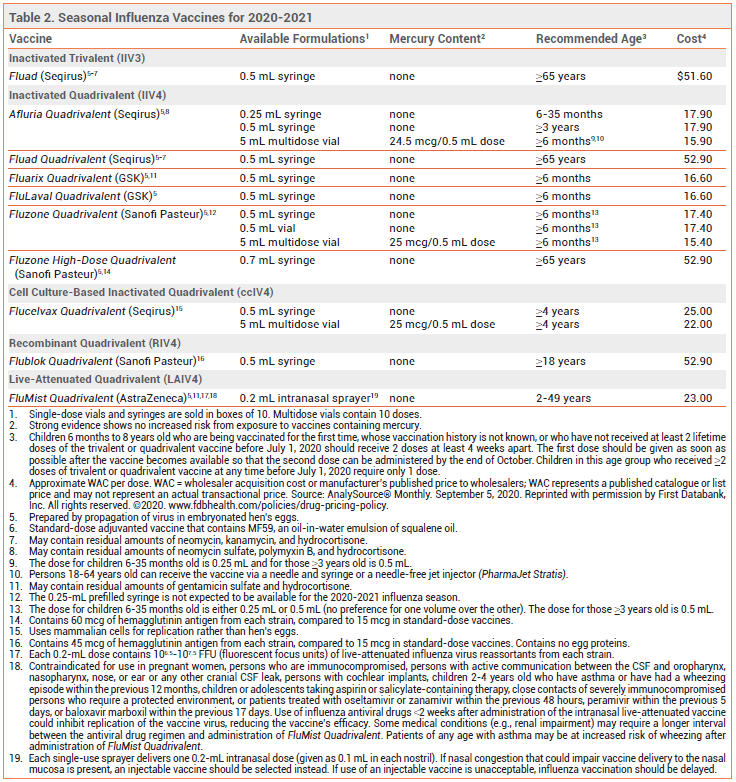
Available vaccines and recommendations for specific patient populations for the 2020 2021 season are listed in tables 2 and 3.

Covid vaccine launch date in russia. The quadrivalent nasal spray vaccine is approved for use in non pregnant individuals 2 years through 49 years old. Regular standard dose trivalent shots. Adjuvanted trivalent influenza vaccine surface antigen inactivated may contain traces of eggs such as ovalbumin or chicken proteins kanamycin and neomycin sulphate formaldehyde cetyltrimethylammonium bromide ctab and hydrocortisone which are used during the manufacturing process see section 43.
Fluad and fluad quadrivalent is a standard dose inactivated influenza flu vaccine manufactured by seqirus that contains an adjuvant. Influenza a h1n1 influenza a h3n2 an influenza b virus. Influenza vaccine products for the 2020 2021 season.
Trivalent flu vaccines protect against three strains of the virus. The who recommends that three influenza strains be included in the trivalent seasonal influenza vaccine. Annual vaccination against influenza a and b viruses is recommended for everyone 6 months old without a contraindication1 vaccination of all eligible persons can reduce the prevalence of influenza illness and symptoms that might be confused with those of covid 19.
Cost effectiveness of introducing an mf59 adjuvanted trivalent influenza vaccine for older adults in argentina van hung nguyen carla vizzotti analia uruena norberto giglio. Quadrivalent seasonal influenza vaccines should contain the three strains recommended for the trivalent vaccine as well as an influenza b virus from the lineage that is not. One influenza ah1n1 one influenza ah3n2 and one influenza b.
During the 2020 2021 influenza season both trivalent fluad and fluad quadrivalent will be available. In summary these are. Adjuvanted trivalent influenza vaccine surface antigen inactivated suspension for injection in pre filled syringe influenza vaccine adjuvanted with mf59c1 patient information leaflet pil by seqirus uk limited.
More information on approved flu vaccines for the 2020 2021 flu season and age indications for each vaccine are available in cdcs table. This adjuvanted vaccine may be abbreviated aiiv3. For those aged 65 and over the adjuvanted trivalent influenza vaccine ativ with the cell based quadrivalent influenza vaccine qivc offered if ativ is unavailable for under 65s at risk including pregnant women offer qivc or as an.
More From Covid Vaccine Launch Date In Russia
- Stonks Meme Template Stinks Meme
- Flu Vaccine Manufacturing Process
- Pfizer Covid Test Results
- Vaccine For 2 Years Old
- Vaccine Is Administered Before Development Of Disease Truefalse
Incoming Search Terms:
- Https Www Mdpi Com 2076 393x 8 3 446 Pdf Vaccine Is Administered Before Development Of Disease Truefalse,
- Influenza Vaccine For 2020 2021 The Medical Letter Inc Vaccine Is Administered Before Development Of Disease Truefalse,
- 3 Vaccine Is Administered Before Development Of Disease Truefalse,
- Pdf Comparative Postmarket Safety Profile Of Adjuvanted And High Dose Influenza Vaccines In Individuals 65 Years Or Older Vaccine Is Administered Before Development Of Disease Truefalse,
- Influenza Vaccine For 2020 2021 The Medical Letter Inc Vaccine Is Administered Before Development Of Disease Truefalse,
- Fluzone High Dose Influenza Vaccine Sanofi Flu Vaccine Is Administered Before Development Of Disease Truefalse,