Adjuvanted Flu Vaccine Canada, Vaccines Free Full Text Experimental Pcep Adjuvanted Swine Influenza H1n1 Vaccine Induced Strong Immune Responses But Did Not Protect Piglets Against Heterologous H3n2 Virus Challenge Html
Adjuvanted flu vaccine canada Indeed lately has been sought by users around us, perhaps one of you. People now are accustomed to using the net in gadgets to view image and video data for inspiration, and according to the name of this post I will talk about about Adjuvanted Flu Vaccine Canada.
- Statement On Seasonal Influenza Vaccine For 2014 2015 Canada Ca
- High Dose Influenza Vaccine For Adults A Review Of Clinical Effectiveness Cost Effectiveness And Guidelines Ncbi Bookshelf
- Https Www Publichealthontario Ca Media Documents F 2020 Fact Sheet Influenza Vaccine 2020 2021 Pdf La En
- Batch Of H1n1 Vaccine Recalled For Severe Reactions The Star
- Opinion Canada S Vaccine Legacy Influenza Polio And Covid 19 The Globe And Mail
- Clinical Trials Network
Find, Read, And Discover Adjuvanted Flu Vaccine Canada, Such Us:
- Canadian Immunization Guide Chapter On Influenza And Statement On Seasonal Influenza Vaccine For 2017 2018 Canada Ca
- Toward A Universal Flu Vaccine Science
- Https Peterboroughpublichealth Ca Wp Content Uploads 2012 09 120925 Flu Monograph Fluad 2012 Pdf
- Health Canada Pulls Distribution Of Novartis Flu Vaccines Cbc News
- Naci Seasonal Influenza Vaccine Statement For 2020 2021 Summary Ccdr 46 5 Canada Ca
If you are searching for Fluenz Tetra Vaccine Contraindications you've reached the right location. We have 101 graphics about fluenz tetra vaccine contraindications adding pictures, photos, pictures, wallpapers, and much more. In these page, we additionally have number of images available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
Oil In Water Emulsion Adjuvants For Pediatric Influenza Vaccines A Systematic Review And Meta Analysis Nature Communications Fluenz Tetra Vaccine Contraindications
Frontiers As03 And Mf59 Adjuvanted Influenza Vaccines In Children Immunology Fluenz Tetra Vaccine Contraindications
Another vaccine specifically designed for seniors is the adjuvanted flu vaccine.

Fluenz tetra vaccine contraindications. As01 b as01 b is an adjuvant suspension used with the antigen component of shingrix vaccine. Children 6 months to less than 9 years of age who have never received the influenza vaccine in a previous influenza season should be given 2 doses of influenza vaccine in the current season with a minimum interval of 4 weeks between doses. Approval in november 2015 had been licensed in 38 countries including canada and 15 european countries.
Mf59 used in flu vaccines in europe since 1997 and in the united states since 2016 has been given to millions of people and has an excellent safety record. The substance added to the adjuvanted flu vaccine is squalene oil also called mf59 which is found naturally in plants and animals. For those who do get sick after getting a flu shot vaccination may reduce.
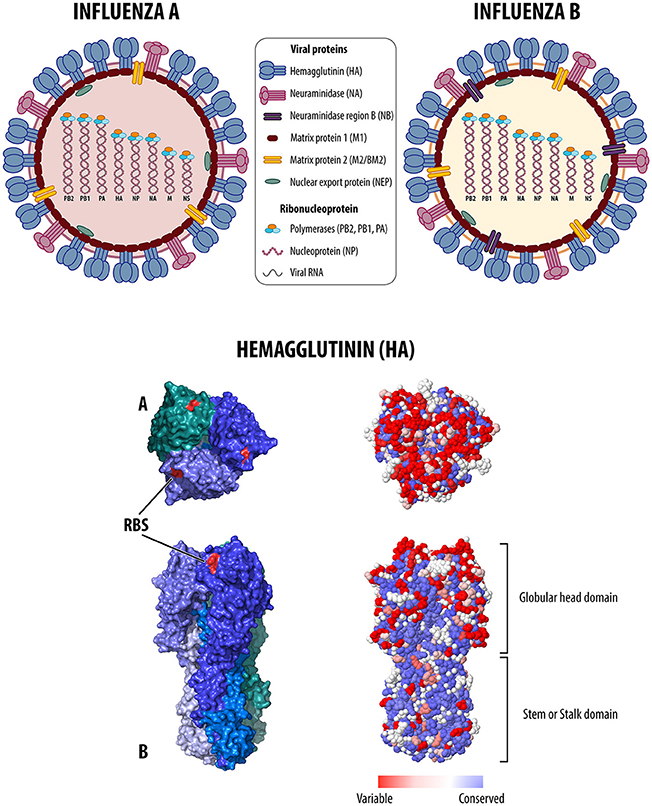
Adjuvanted inactivated influenza vaccine iiv adj the adjuvanted inactivated influenza vaccine iiv adj currently authorized for use in canada is a subunit iiv that contains the adjuvant mf59 which is an oil in water emulsion composed of squalene as the oil phase that is stabilized with the surfactants polysorbate 80 and sorbitan triolate in. People 65 years and older can get any flu vaccine approved for use in that age group with no. In february 2011 fluad novartis a trivalent inactivated subunit influenza vaccine tiv adjuvanted with mf59c1 was authorized in canada for use in adults 65 years of age and older for active immunization against influenza caused by specific strains of influenza virus contained in the vaccine.
Recombinant flu vaccines are produced using a method that does not require an egg grown vaccine virus. Adjuvanted inactivated influenza vaccine iiv adj the adjuvanted inactivated influenza vaccine iiv adj currently authorized for use in canada is a trivalent subunit iiv that contains the adjuvant mf59 which is an oil in water emulsion composed of squalene as the oil phase that is stabilized with the surfactants polysorbate 80 and sorbitan. Flu vaccine with adjuvant brand names fluad and fluad quadrivalent.
We estimated the relative vaccine effectiveness rve of a trivalent high dose hd iiv3 versus an adjuvanted trivalent influenza vaccine aiiv3 in seniors for respiratory related hospitalizations. In italy in 1997 and at the time of its us. It is the first seasonal influenza vaccine.
Adjuvanted flu vaccine is made with an ingredient added to a vaccine that helps create a stronger immune response and is licensed specifically for people 65 years and older. This gives an extra boost. Adults 65 years and older seniors experience more complications following influenza infection than younger adults.
Adults and children 9 years of age and older should receive 1 dose of influenza vaccine each year.
More From Fluenz Tetra Vaccine Contraindications
- Covid Vaccine How Does It Work
- Covid Vaccine Data
- Pfizer Covid Vaccine Study Volunteer
- Varicella Zoster Vaccine Contraindications
- Covid Vaccine Russia Update
Incoming Search Terms:
- Pdf Did Narcolepsy Occur Following Administration Of As03 Adjuvanted A H1n1 Pandemic Vaccine In Ontario Canada A Review Of Post Marketing Safety Surveillance Data Covid Vaccine Russia Update,
- Opinion Canada S Vaccine Legacy Influenza Polio And Covid 19 The Globe And Mail Covid Vaccine Russia Update,
- H1n1 Flu Shots Likely Tied To Increase In Paralysis Syndrome Cbc News Covid Vaccine Russia Update,
- Toward A Universal Flu Vaccine Science Covid Vaccine Russia Update,
- Canada Approves Cell Based Quadrivalent Influenza Vaccine Vax Before Travel Covid Vaccine Russia Update,
- Cost Effectiveness Of Influenza Vaccine Strategies For The Elderly In South Korea Covid Vaccine Russia Update,