Vaccine Trial Phases Ppt, Understanding Clinical Trial Terminology What S A Phase 1 2 Or 3 Clinical Trial Concert Pharmaceuticals
Vaccine trial phases ppt Indeed recently is being hunted by users around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view image and video data for inspiration, and according to the name of this article I will talk about about Vaccine Trial Phases Ppt.
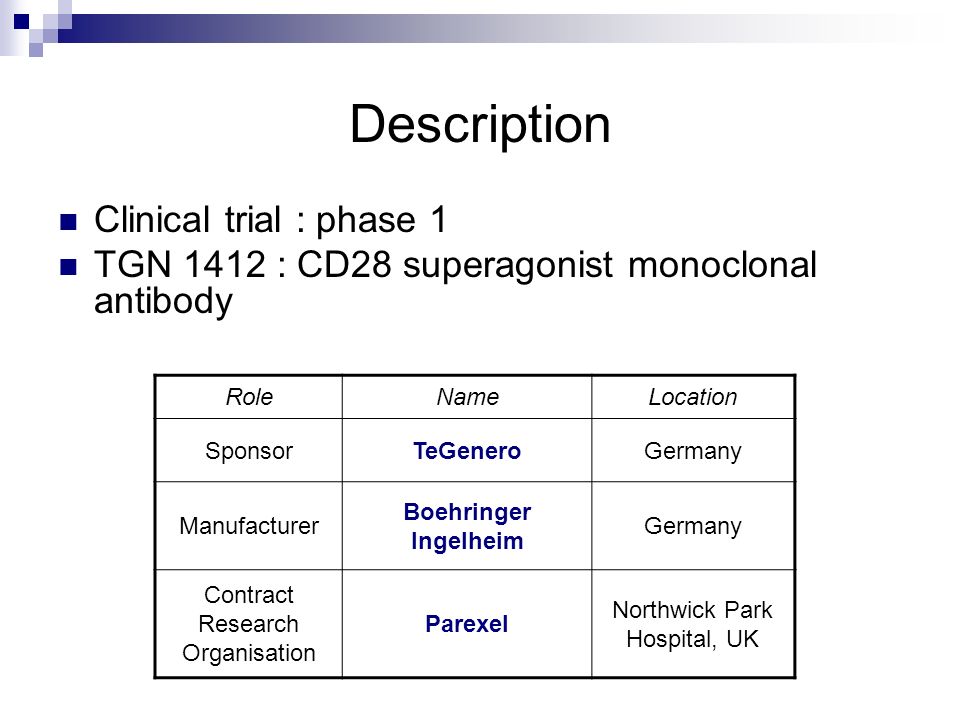
- Clinical Trial Phases
- The Drug Review Approval Process In Canada An Eguide Spharm Drug Regulatory Experts
- Key Design Considerations For Adaptive Clinical Trials A Primer For Clinicians The Bmj
- Clinical Trial Powerpoint Template Sketchbubble
- Clinical Trials And Drug Development
- Gtl001 A Therapeutic Vaccine For Women Infected With Human Papillomavirus 16 Or 18 And Normal Cervical Cytology Results Of A Phase I Clinical Trial Clinical Cancer Research
Find, Read, And Discover Vaccine Trial Phases Ppt, Such Us:
- Clinical Trials And Drug Development
- Revisiting The Correlate Of Reduced Hiv Infection Risk In The Rv144 Vaccine Trial Journal Of Virology
- Regulatory Changes In China S Biopharma Market Deloitte Insights
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gct3chduhhqembjzhsqjjivqvxhenznbcom6uiqwsb0gmn2obkiw Usqp Cau
- Challenges And Opportunities In Adapting Clinical Trial Design For Immunotherapies Clinical Cancer Research
If you are searching for Moderna Stock Price Forecast you've come to the perfect location. We have 104 graphics about moderna stock price forecast adding images, pictures, photos, backgrounds, and more. In such web page, we additionally have variety of images available. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, translucent, etc.
Phase 2 trial of two vaccines to prevent ebola in liberia sb kennedy hc lane et al.

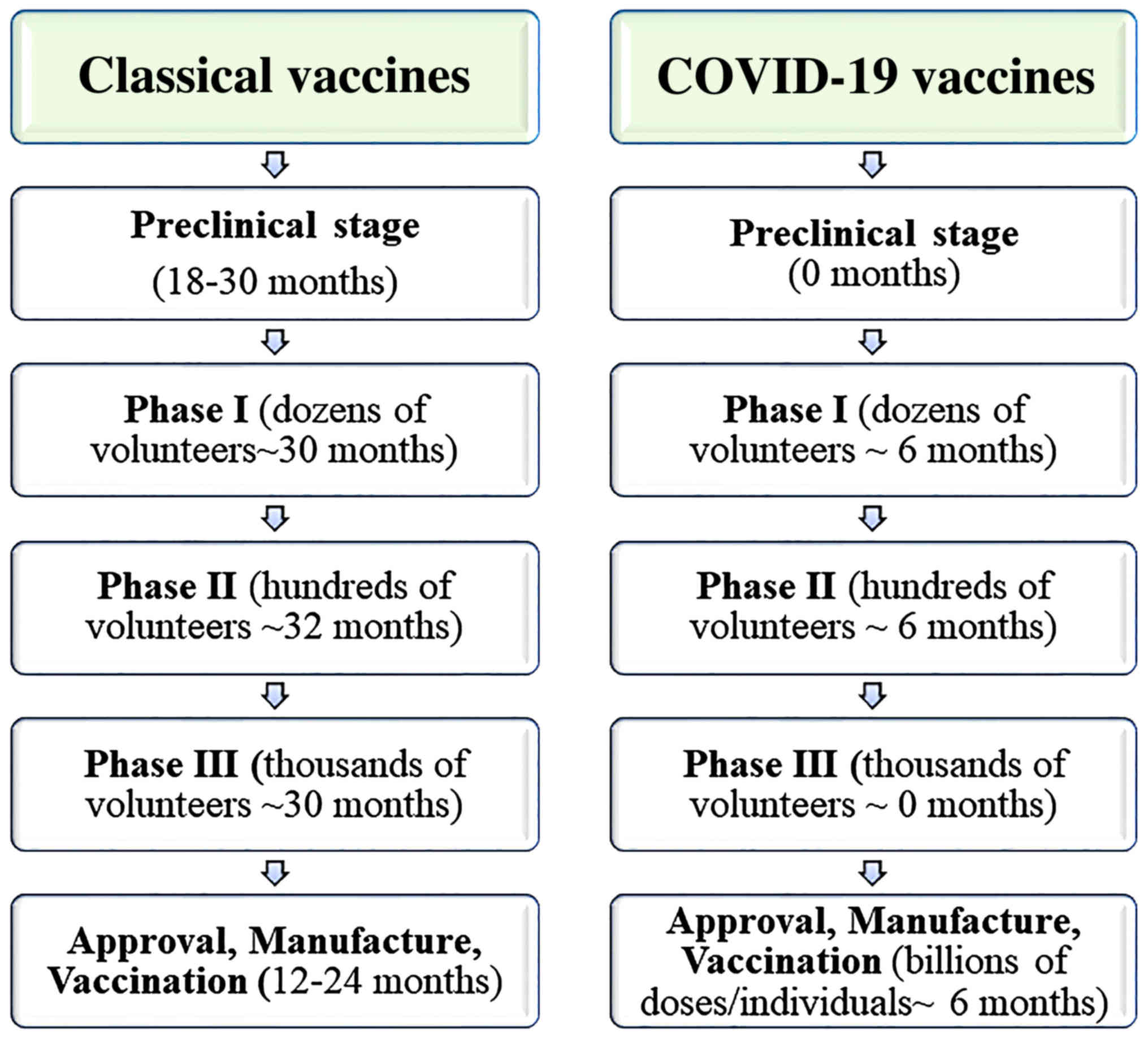
Moderna stock price forecast. Overview pre clinical evaluation phases of a vaccine trial phase i phase ii phase iii phase iv emergency approval post licensure vaccine safety activities notable vaccine safety issues improving global monitoring conclusion 3. A vaccine candidate drug is first identified through preclinical evaluations that could involve high throughput screening and selecting the proper antigen to invoke an immune response. The full development pathway for an effective vaccine for sars cov2 will require that industry government and academia collaborate in unprecedented ways each adding their individual strengths.
Phase i vaccine clinical trials are small typically enrolling 30 to 100 human. Phase iii of a clinical trial usually involves up to 3000 participants who have the condition that the new medication is meant to treat. Outline introduction vaccine development.
In phase iii the vaccine is given to thousands of people and tested for efficacy and safety. The research team keeps a close eye on the people and watches for any severe side effects. We further discuss a collaborative platform for conducting harmonized randomized controlled vaccine efficacy trials.
Trials in this phase can last for several years. For the prevail i study group volume 375 october 13 2016 number 15 a randomized controlled trial of zmapp for ebola virus infection the prevail il writing group for the multi national prevail il study team. Clinical trial phases 345 by danish ibrahim jasnaik danish jasnaik.
1 s20 s0016003213003104 main ramalakshmi vijay. In phase ii the clinical study is expanded and vaccine is given to people who have characteristics such as age and physical health similar to those for whom the new vaccine is intended. A sponsor who wishes to begin clinical trials with a vaccine must submit an investigational new drug.
Safety is the main concern. Volume 380 march 7 2019. Phase i trials are also looking at what the drug does to the body and what the body does with the drug.
A vaccine trial is a clinical trial that aims at establishing the safety and efficacy of a vaccine prior to it being licensed. Clinical research ppt malay singh. Because of the small numbers of people in phase i studies rare side effects may not be seen until later phases of trials.
Vaccine trials dr sahil kumar 2. Rt davey jr d malvy et al. Phases of clinical trials 9 phases of clinical trials pharmaceutical biotechnology 9 10.
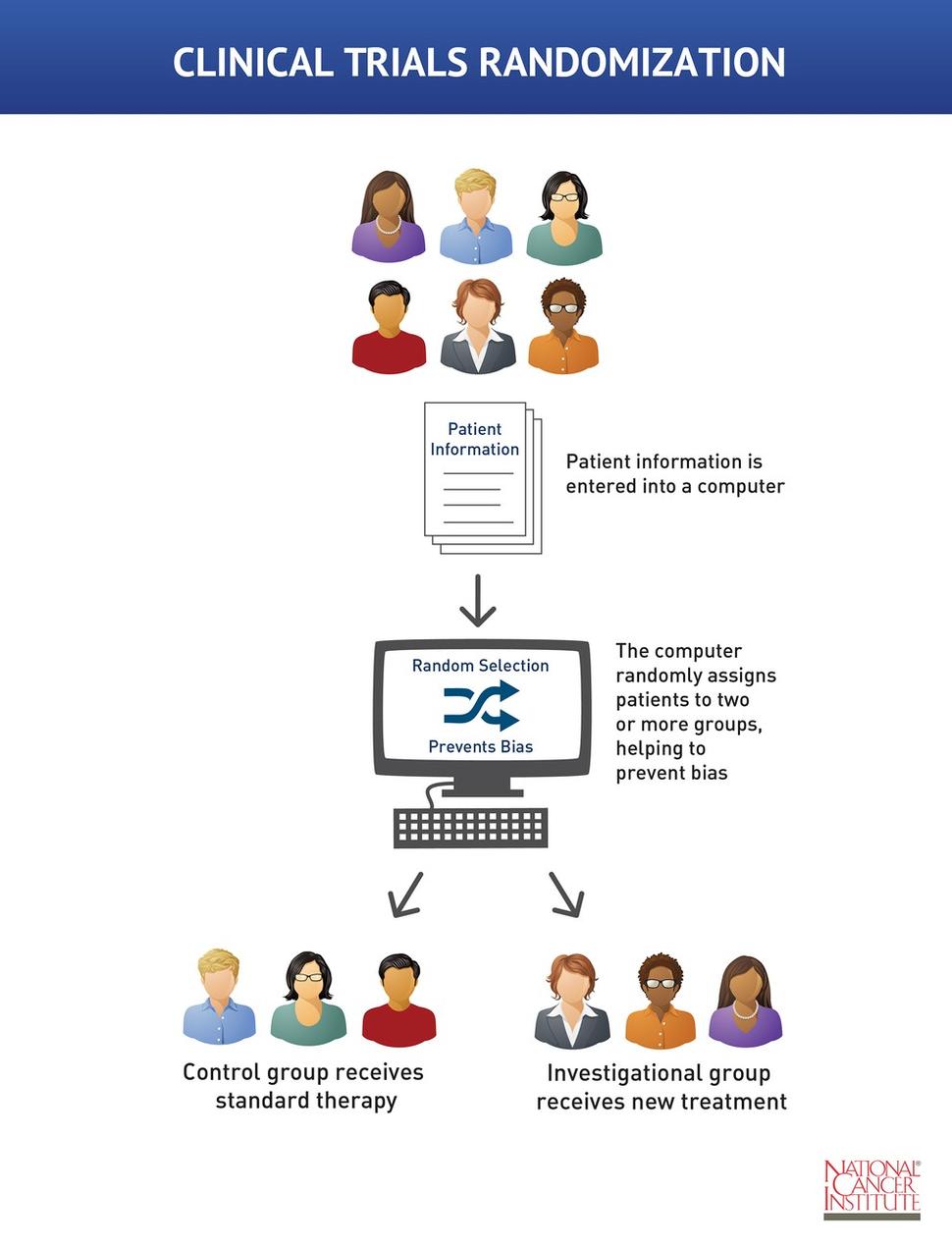
They are usually double blind.
More From Moderna Stock Price Forecast
- Downward Facing Dog Variations Yoga
- Hpv Vaccine History Canada
- Dowagers Hump Correction Before And After
- Coronavirus Vaccine Usa 2021
- Covid Vaccine Govuk
Incoming Search Terms:
- A Strategic Approach To Covid 19 Vaccine R D Science Covid Vaccine Govuk,
- Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International Covid Vaccine Govuk,
- P Hacking In Clinical Trials And How Incentives Shape The Distribution Of Results Across Phases Pnas Covid Vaccine Govuk,
- Preclinical Development Wikipedia Covid Vaccine Govuk,
- Ongoing Clinical Trials For The Management Of The Covid 19 Pandemic Trends In Pharmacological Sciences Covid Vaccine Govuk,
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsiay3d3gxlb7nlo0u Zkbxgqe5 Uznktgq7h70ll2nw2prjsap Usqp Cau Covid Vaccine Govuk,